Preparation technology of carboxybenzaldehyde
A technology for the preparation of o-carboxybenzaldehyde, which is applied in the preparation of carboxylate salts, the preparation of organic compounds, organic chemistry, etc., can solve the problems of low yield of o-carboxybenzaldehyde, cumbersome preparation process steps, and poor controllability. Achieve the effects of convenient operation, simple preparation process steps and sufficient reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
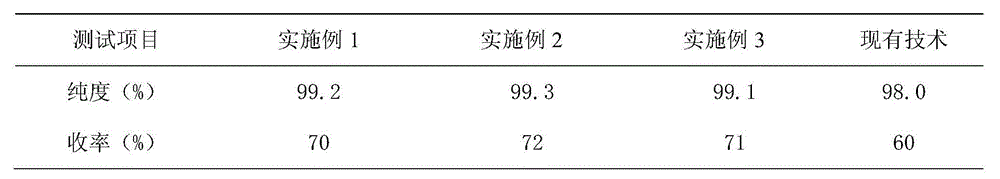
[0018] Embodiment 1: the preparation technology of a kind of o-carboxybenzaldehyde of the present embodiment comprises the following steps:
[0019] In terms of parts by mass, add 60 parts of chloroform and 120 parts of phthalide into the reaction kettle, heat to 105°C, pressurize to 8Mpa, add 100 parts of bromine water with a concentration of 80% dropwise within 1h and stir, and heat to 115 Reflux at 120°C for 1 hour, and pass nitrogen gas into the reactor for 1 hour at 120°C to make the reaction fully and remove hydrogen bromide gas, evaporate to dryness under reduced pressure to remove residual impurities, cool to 8°C, and dry at 20°C , to obtain 3-bromophthalide;
[0020] Add 40 parts of deionized water into the hydrolysis kettle, heat to 90°C, put in the 3-bromophthalide, heat in a water bath at 100°C for 2 hours, cool to 10°C, crystals are precipitated, and the wet product of o-carboxybenzaldehyde is obtained by suction filtration. Add o-carboxybenzaldehyde wet product ...
Embodiment 2
[0021] Embodiment 2: the preparation technology of a kind of o-carboxybenzaldehyde of the present embodiment comprises the following steps:
[0022] In terms of parts by mass, add 80 parts of chloroform and 120 parts of phthalide into the reaction kettle, heat to 105°C, pressurize to 8Mpa, add 110 parts of bromine water with a concentration of 80% dropwise within 1.2h, stir, and heat to Reflux at 115°C for 1 hour, and at 120°C, pass nitrogen gas into the reaction kettle for 1.2 hours to make the reaction fully and remove hydrogen bromide gas, evaporate to dryness under reduced pressure to remove residual impurities, cool to 8°C, and at 20°C Drying is carried out to obtain 3-bromophthalide;
[0023] Add 60 parts of deionized water into the hydrolysis kettle, heat to 90°C, put in the 3-bromophthalide, heat in a water bath at 100°C for 2 hours, cool to 10°C, precipitate crystals, and obtain the wet product of o-carboxybenzaldehyde by suction filtration. Add o-carboxybenzaldehyde...
Embodiment 3
[0024] Embodiment 3: the preparation technology of a kind of o-carboxybenzaldehyde of the present embodiment comprises the following steps:
[0025] In terms of parts by mass, add 100 parts of chloroform and 120 parts of phthalide into the reaction kettle, heat to 105°C, pressurize to 8Mpa, add 120 parts of bromine water with a concentration of 80% dropwise within 1.5h, stir, and heat to Reflux at 115°C for 1 hour, and at 120°C, pass nitrogen gas into the reaction kettle for 1.5 hours to make the reaction fully and remove hydrogen bromide gas, evaporate to dryness under reduced pressure to remove residual impurities, cool to 8°C, and in 20°C Drying is carried out to obtain 3-bromophthalide;
[0026] Add 80 parts of deionized water into the hydrolysis kettle, heat to 90°C, put in the 3-bromophthalide, heat in a water bath at 100°C for 2 hours, cool to 10°C, precipitate crystals, and obtain the wet product of o-carboxybenzaldehyde by suction filtration. Add o-carboxybenzaldehyd...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com