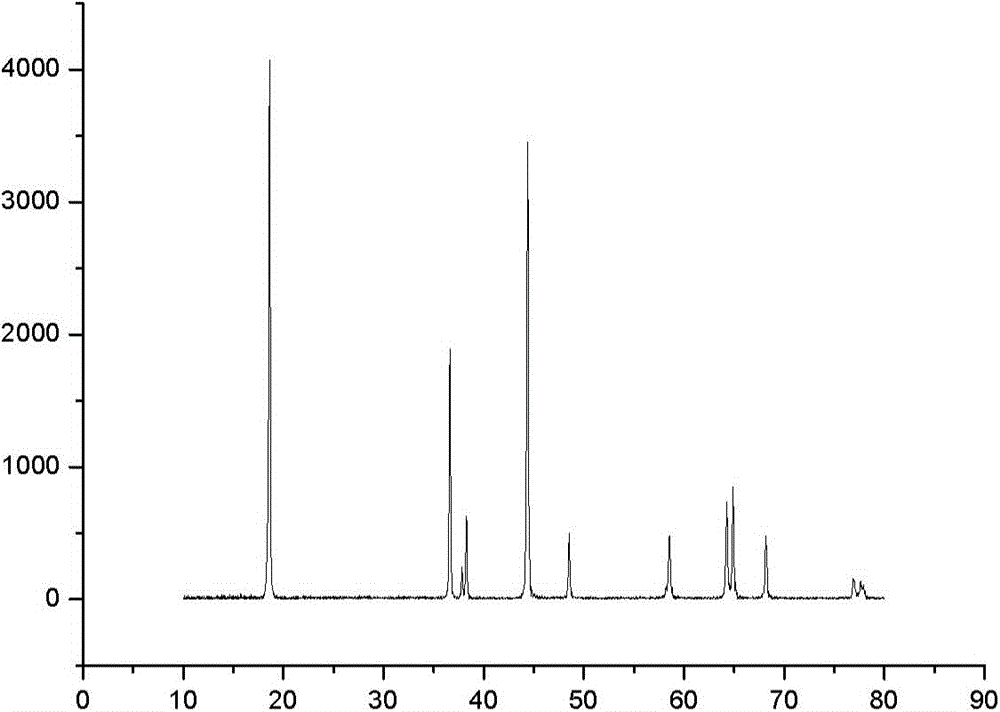
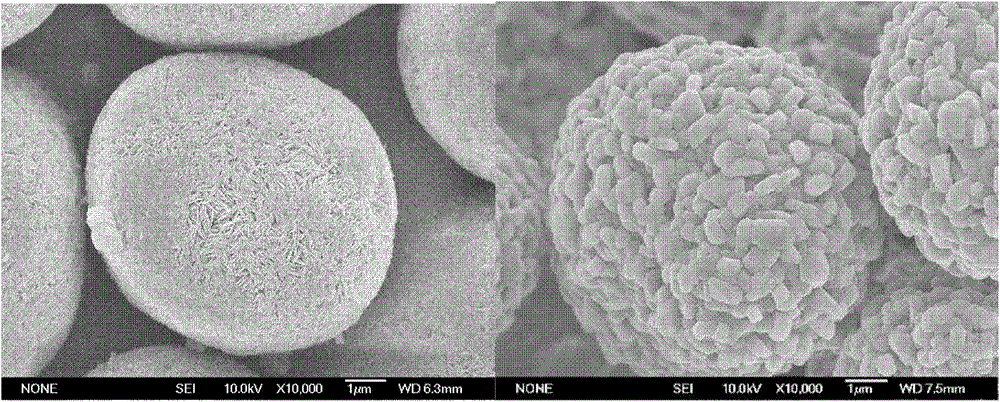
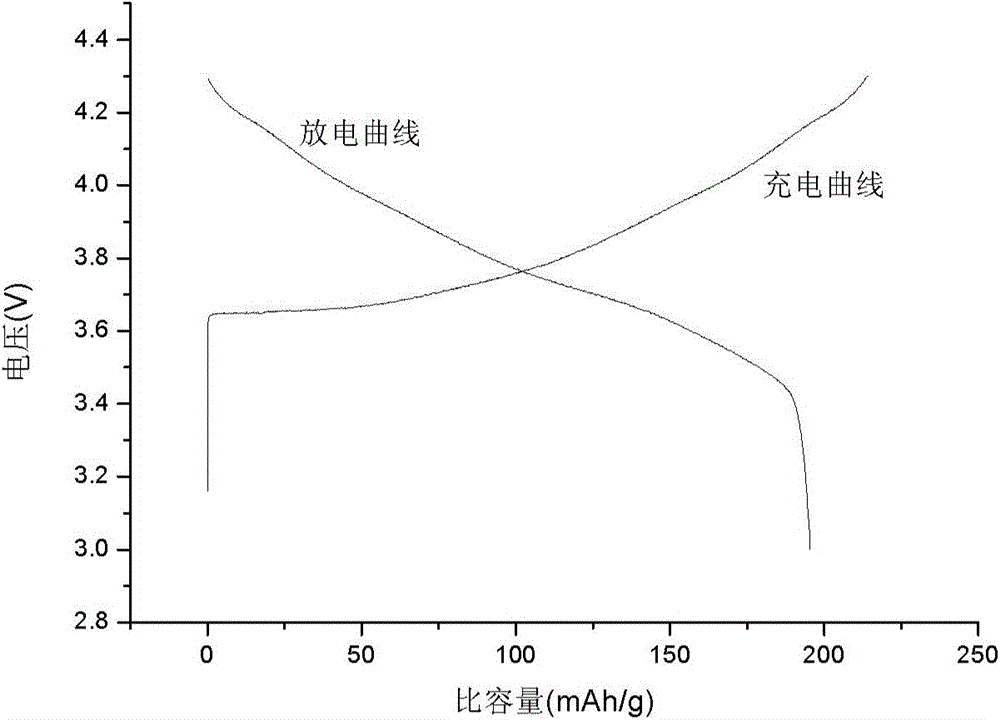
High-density spherical Ni-Co lithium aluminate material and preparation of precursor of the material
A technology of nickel-cobalt-lithium-aluminate and precursors, which is applied in the field of preparation of high-density spherical nickel-cobalt-lithium aluminate materials and their precursors, can solve problems such as low tap density, irregular shape, and poor electrical properties. Achieve the effects of increased charge and discharge capacity, improved first-time efficiency, and simple and easy methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The method for preparing a nickel-cobalt-aluminum precursor material and the method for preparing a nickel-cobalt-aluminum-aluminate material using the nickel-cobalt-aluminum precursor material are described in detail below.
[0038] The method for preparing nickel-cobalt-aluminum precursor material of the present invention comprises: step (1) preparation contains the solution A of nickel (II) salt and cobalt (II) salt; Solution B containing aluminum complex; Alkaline solution C, wherein aluminum complex solution B is prepared by dissolving inorganic aluminum salts and at least two types of complexing agents in water; step (2) injecting the prepared solution A and solution B into the reactor synchronously at a controlled flow rate , so that the nickel-cobalt-aluminum ion molar ratio reaches the nickel-cobalt-aluminum ion molar ratio in the lithium-nickel-cobalt-aluminate material, while adding solution C, to keep the pH value of the mixed system between 10-12.7; and step...
Embodiment 1
[0063] 1700g NiSO 4 ·6H 2 O and 330g CoSO 4 ·7H 2 O was dissolved in 4L deionized water to form solution A, and 150g Al(NO 3 ) 3 9H 2O and 57g of triethanolamine, 150g of EDTA, and 42g of anhydrous sulfosalicylic acid were dissolved in 2L of deionized water. After adding sulfuric acid to adjust the pH to 3, the solution was placed in a 75-degree water bath heating stirrer and stirred for 2 hours to form an aluminum complex solution. B; prepare 4L of 6M sodium hydroxide solution, add 1.2L of 28% ammonia water and mix well as alkaline solution C. Add 2L of mother liquor into the 15L reactor, heat it to 50°C, and add solutions A and B in parallel, wherein the flow rate of solution A is controlled at 0.2L / h, the flow rate of solution B is controlled at 0.1L / h, and the alkali solution C is opened at the same time , adjust the flow rate of the alkaline solution to control the pH value of the reactor system between 12.0-12.5, stop adding the alkaline solution after solution A a...
Embodiment 2
[0066] 1700g NiSO 4 ·6H 2 O and 220g CoSO 4 ·7H 2 O was dissolved in 4L deionized water to form solution A, and 300g Al(NO 3 ) 3 9H 2 O, 105 g of diethanolamine, and 115 g of tartaric acid were dissolved in 2 L of deionized water, and after adding sulfuric acid to adjust the pH=2, the solution was placed in a 50-degree water bath heating stirrer and stirred for 2 hours to form aluminum complex solution B; Sodium hydroxide solution, after adding 3L of 28% ammonia water and mixing, it was used as alkaline solution C. Add 2L of mother liquor into the 15L reactor, heat it to 50°C, control the flow rate of solutions A and B at 0.2L / h and add solutions A and B in parallel, and at the same time open the alkali solution C to adjust the flow rate of the alkali solution to control the reactor system When the pH value is between 11.5-12.0, stop adding the alkali solution after solution A and solution B are all added to the reactor, and after solid-liquid separation, use deionized w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Tap density | aaaaa | aaaaa |
| Charging capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com