Active fluorescence detection method of anthropogenic silent information regulatory factor 6 (Sirtuin6)
A technology for regulating factors and silencing, applied in the field of pharmacy, can solve the problems of misdirection and the decrease in the affinity of substrate peptides and SIRT6, and achieve the effect of accurate screening effect, tight binding and low cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 of the present invention: Activity fluorescence detection method of human sirtuin 6:
[0028] Reagents and Instruments
[0029] All reagents were purchased from Aldrich or Acros company of analytical grade without further processing; VARIAN INOVA 400M Hz nuclear magnetic resonance instrument; liquid mass spectrometer was Shimadzu-LC20A and Thermo LCQ FLEET mass spectrometer, and the analytical column was Sprite TARGA C18 column (40 × 2.1 mm, 5 μm, Higgins Analytical, Inc.), the detection wavelength is 215 and 280 nanometers, using the binary gradient elution method of 0.1% formic acid solution and 0.1% formic acid acetonitrile solution; BIO-TEK? Synergy H4 multifunctional enzyme label instrument (excitation wavelength 336 nm, emission wavelength 490 nm).
[0030] Synthesis and purification of peptides
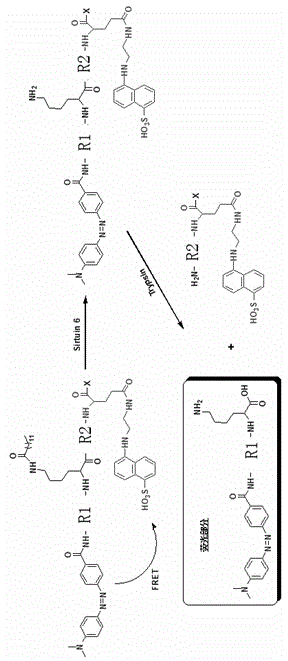
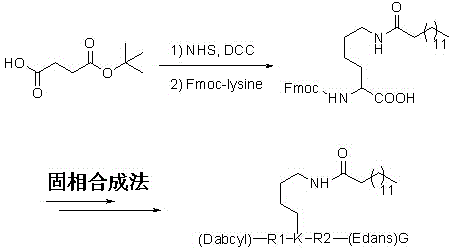
[0031] Synthetic route such as image 3 As shown, long-chain fatty acylated peptides and other peptides were synthesized by Fmoc-Wang resin using sta...
Embodiment 2
[0054] Embodiment 2 of the present invention: the screening method of Sirtuin 6 regulator:
[0055] (Dabcyl)ISGASEK(Myristoyl)DIVHSE(Edans)G Screening for Inhibitors of SIRT6
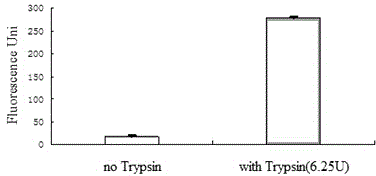
[0056] The steps here are the same as those described above (fluorescence experiment of (Dabcyl)ISGASEK(Succinyl)DIVHSE(Edans)G polypeptide under the catalysis of SIRT6), except that different compounds to be tested (both at a concentration of 30uM) were added to the reaction. In the first step In the reaction, SIRT6 was added at the end to start the reaction. Experimental results such as Figure 7 shown.
[0057] Determination of IC50 of SIRT6 inhibitors
[0058] The steps here are the same as those described above ((Dabcyl)ISGASEK(Succinyl)DIVHSE(Edans)G screening SIRT6 inhibitors), except that different concentrations of Nicotinamide (50, 100, 200, 500, 1000, 2000uM). Experimental results such as Figure 8 shown.
[0059] according to Figure 7 It was learned that no SIRT6 and only SIRT6 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com