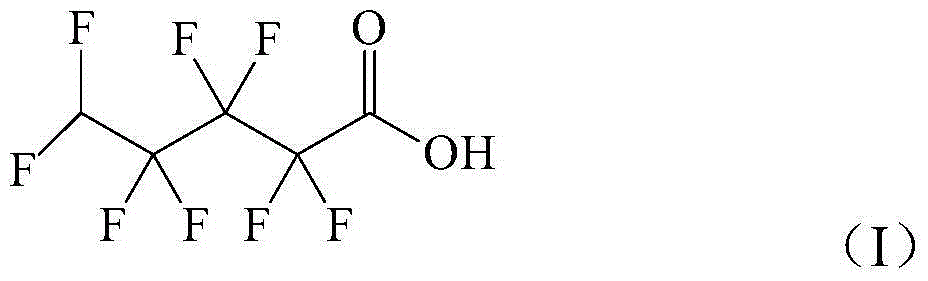
Method for preparing octafluorovaleric acid under catalytic oxidation action of oxydol
A technology of catalytic oxidation and octafluorovaleric acid, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate and other directions, can solve the problems of high price, insufficient selectivity, three wastes, etc., and achieves reduction of production cost, The effect of eliminating pollution sources and low prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Weigh 136g (1.2mol) of hydrogen peroxide, 3.96g (0.012mol) of sodium tungstate and 3.87g (0.012mol) of tetrabutylammonium bromide with a content of 30% and place them in a magnetic stirring, reflux condenser, constant pressure feeding In a 1L flask with a funnel, an oil bath (normal temperature) and a thermometer, stir in an oil bath at 35°C for about 30 minutes to fully dissolve sodium tungstate and tetrabutylammonium bromide.
[0032] After raising the temperature to 85-90°C, 116 g (0.5 mol) of octafluoropentanol, the raw material, was added dropwise, and the vigorous stirring (500 r / min) was continued for 8 hours until the reaction was completed. Then continue to heat up the reaction solution and boil it, and check it with starch potassium iodide test paper until no blue color appears. Add 2.37g of BaCO 3 Powder, stirred for 1h, cooled slightly and filtered to remove solid precipitate. The colorless filtrate was concentrated to remove water by distillation under re...
Embodiment 2
[0035] Take by weighing 30% hydrogen peroxide 136g (1.2mol), 3g (0.012mol) tungstic acid (H 2 WO 4 ) and 1.08g (0.012mol) of oxalic acid were placed in a 1L flask equipped with magnetic stirring, reflux condenser, constant pressure addition funnel, oil bath (normal temperature) and thermometer, stirred in an oil bath at 40°C for 20min, fully Dissolve tungstic acid and oxalic acid.
[0036] After raising the temperature to 85-90°C, 116 g (0.5 mol) of octafluoropentanol, the raw material, was added dropwise, and the vigorous stirring (500 r / min) was continued for 8 hours until the reaction was completed. Then continue to heat up the reaction solution and boil it, and check it with starch potassium iodide test paper until no blue color appears. Add 2.37g of BaCO 3 Powder, stirred for 1h, cooled slightly and filtered to remove solid precipitate. The colorless filtrate was concentrated by distillation under reduced pressure to remove water, and then extracted three times with d...
Embodiment 3
[0039] Weigh 136g (1.2mol) of hydrogen peroxide with a content of 30%, 3.96g (0.012mol) of sodium tungstate and 3.73g (0.011mol) of tetrabutylammonium bisulfate and place them in a magnetic stirring, reflux condenser, constant pressure feeding In a 1L flask with a funnel, an oil bath (normal temperature) and a thermometer, stir in an oil bath at 35°C for about 30 minutes to fully dissolve sodium tungstate and tetrabutylammonium bisulfate.
[0040] After raising the temperature to 85-90°C, 139.2 g (0.6 mol) of octafluoropentanol, the raw material, was added dropwise, and the vigorous stirring (500 r / min) was continued for 8 hours until the reaction was completed. Then continue to heat up the reaction solution and boil it, and check it with starch potassium iodide test paper until no blue color appears. Add 2.37g of BaCO 3 Powder, stirred for 1h, cooled slightly and filtered to remove solid precipitate. The colorless filtrate was concentrated to remove water by distillation un...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com