A kind of zwitterionic amino polyfluorene derivative and its synthesis method and application
A technology of ionic amines and derivatives, applied in the field of zwitterionic amine-based polyfluorene derivatives and their synthesis, to achieve the effects of easy processing and film formation, optimization, pollution reduction, and good environmental adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
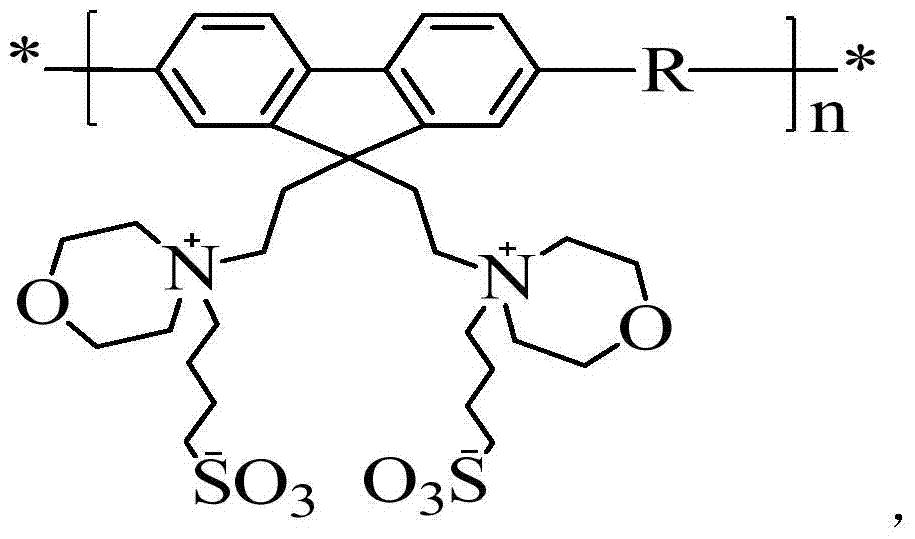
[0035] 1) Synthesis of 2,7-dibromo-9,9-bis(2-ethylmorpholine)fluorene (M1)
[0036] 2,7-Dibromofluorene (6.5g, 20mmol), 4-(2-chloroethyl)morpholine hydrochloride (11.2g, 60mmol) and potassium hydroxide (11.3g, 200mmol) were dissolved in 200mL THF , reacted at 80oC for 48h in an anhydrous and oxygen-free Ar environment. After the reaction, the solvent tetrahydrofuran was distilled under reduced pressure, and the product was first purified by column chromatography (silica gel, ethyl acetate: methanol = 10:1 as the eluent), and the milky white solid was recrystallized in petroleum ether, and finally 5 g of white crystals were obtained. , the yield was 45%.
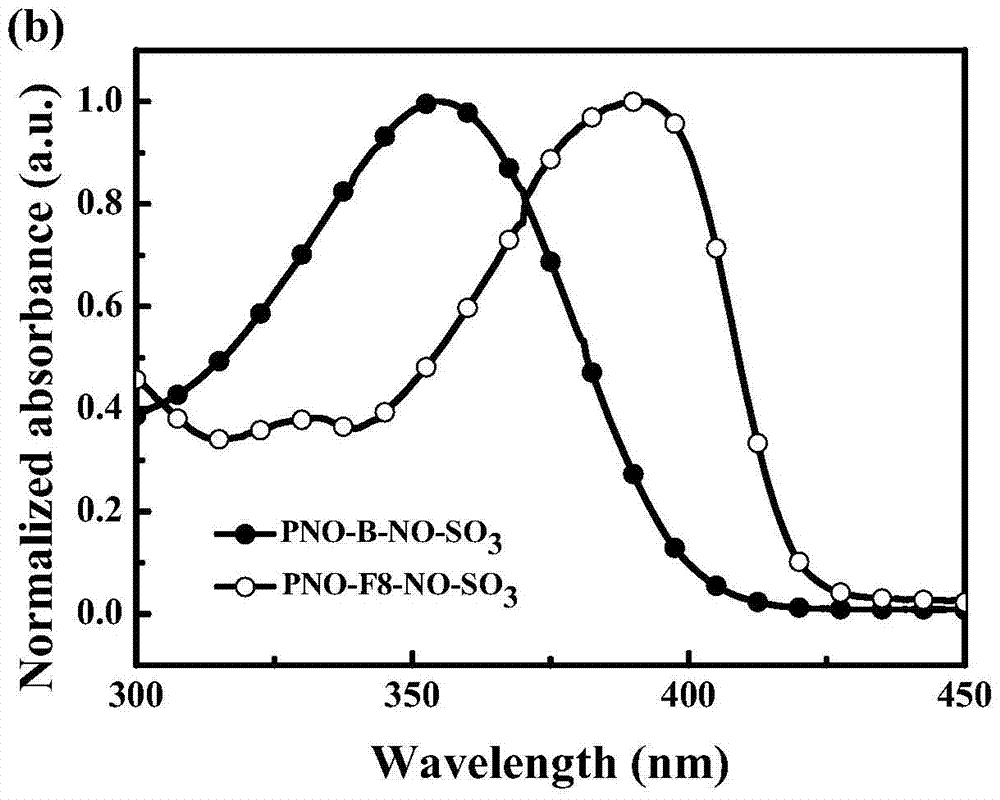
[0037] 2) PFNO-B-FNO-SO 3
[0038] In a 50mL three-necked flask, M1 (550mg, 1mmol), 1.4-dibenboronic acid (169mg, 1mmol), tetraphenylpalladium phosphide (15mg, 0.01mmol), potassium carbonate (1.38g, 10mmol) were added to 10mL of toluene 5mL water mixture. The reaction system was refluxed for 48 h under the protection of ...
example 2
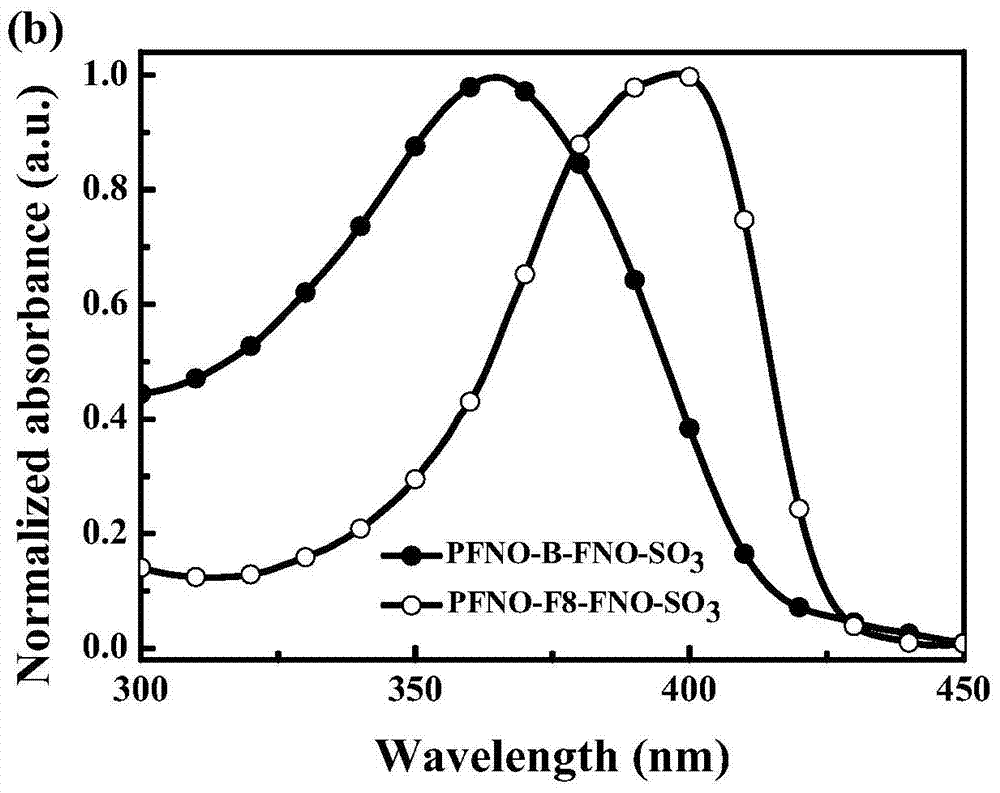
[0040] 1) Synthesis of 2,7-dibromo-9,9-bis(2-ethylmorpholine)fluorene (M1)
[0041] 2,7-Dibromofluorene (6.5g, 20mmol), 4-(2-chloroethyl)morpholine hydrochloride (11.2g, 60mmol) and potassium hydroxide (11.3g, 200mmol) were dissolved in 200mL THF , reacted at 80oC for 48h in an anhydrous and oxygen-free Ar environment. After the reaction, the solvent tetrahydrofuran was distilled under reduced pressure, and the product was first purified by column chromatography (silica gel, ethyl acetate: methanol = 10:1 as the eluent), and the milky white solid was recrystallized in petroleum ether, and finally 5 g of white crystals were obtained. , the yield was 45%.
[0042] 2) Synthesis of 2,7-dibromo-9,9-dioctylfluorene (M2)
[0043] Put 2,7-dibromofluorene (5g, 15.5mmol), tetrabutylammonium bromide (0.1g, 0.31mmol), and 200mL dimethyl sulfoxide in a three-necked flask under vigorous stirring to form a suspension, drop 50wt % sodium hydroxide aqueous solution 5mL, after reacting for 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com