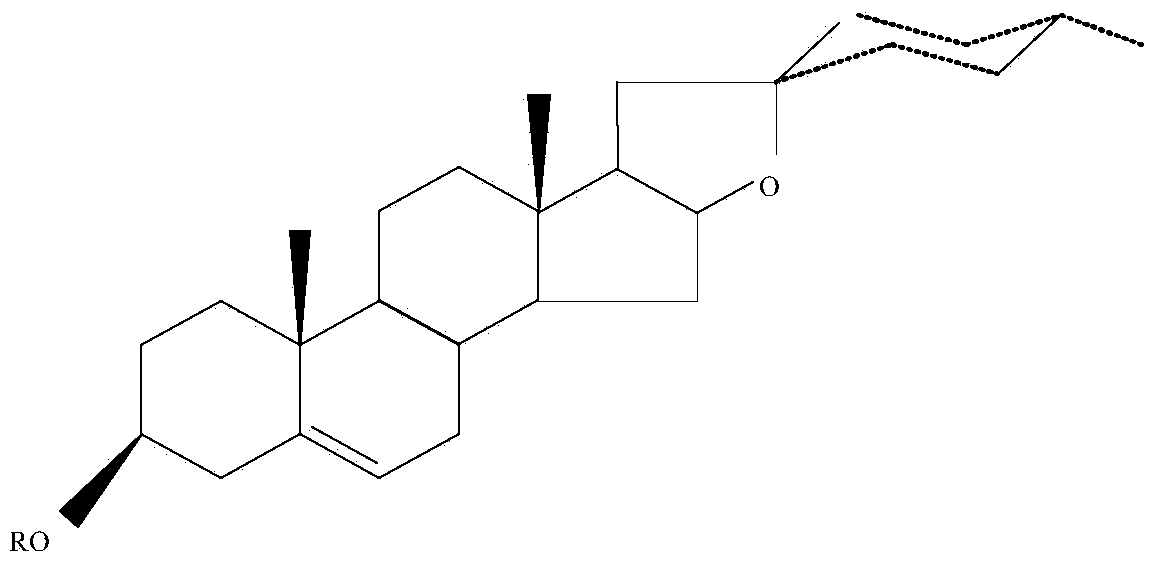
Diosgenin amino acid derivative and application thereof to antitumor drug
A technology of diosgenin and derivatives, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0034] Preparation of L-phenylalanine diosgenin ester:
[0035]Weigh 0.1000 g (0.24 mmol) of diosgenin, 0.1280 g (0.48 mmol) of N-BOC-L phenylalanine, 0.1000 g (0.48 mmol) of DCC, 0.058 g (0.48 mmol) of DMAP, and an appropriate amount of molecular sieves into 50 mL of reaction Add 25 mL of dichloromethane to the bottle, place the reaction mixture on a magnetic stirrer at room temperature to accelerate the reaction, and use TLC (petroleum ether-ethyl acetate = 4:1 v:v) to detect the reaction progress, and each Check every 15 minutes. During the reaction process, new substances will be produced, and the reaction is stopped when the reaction is complete, and the reaction time is about 2 h. Filter the liquid in the reaction bottle, evaporate the solvent under reduced pressure until 2-3 mL of liquid remains in the round bottom flask to stop the reaction, pour the remaining liquid into an evaporating dish, and evaporate in a water bath (temperature 45 ℃) in a fume hood , evaporate...
example 2
[0037] Preparation of L-alanine diosgenin ester:
[0038] Weigh 0.1000 g (0.24 mmol) of diosgenin, 0.0908 g (0.48 mmol) of N-BOC-L-alanine, 0.1000 g (0.48 mmol) of DCC, 0.058 g (0.48 mmol) of DMAP, and an appropriate amount of molecular sieves into 50 mL of reaction 25 mL of dichloromethane was added to the bottle, and the reaction mixture was reacted under magnetic stirring at room temperature, and the reaction progress was detected by TLC (petroleum ether-ethyl acetate=4:1 v:v) every 15 min. During the reaction process, new substances will be produced, and the reaction is stopped when the reaction is complete, and the reaction time is about 24 h. Filter the liquid in the reaction bottle, evaporate the solvent under reduced pressure until 2-3 mL of liquid remains in the round bottom flask to stop the reaction, pour the remaining liquid into an evaporating dish, and evaporate in a water bath (temperature 45 ℃) in a fume hood , evaporated until it was mucus, and purified by si...
example 3
[0040] Preparation of L-proline diosgenin ester:
[0041] Weigh 0.1000 g (0.24 mmol) of diosgenin, 0.1033 g (0.48 mmol) of N-BOC-L-proline, 0.1000 g (0.48 mmol) of DCC, 0.058 g (0.48 mmol) of DMAP, and appropriate amount of molecular sieves into 50 mL of reaction Add 25 mL of tetrahydrofuran to the bottle, and the reaction mixture reacts under magnetic stirring at room temperature. TLC (petroleum ether-ethyl acetate=4:1 v:v) is used to detect the reaction progress, and the detection is performed every 15 min. During the reaction process, new substances will be produced, and the reaction is stopped when the reaction is complete, and the reaction time is about 2 h. Filter the liquid in the reaction bottle, evaporate the solvent under reduced pressure until 2-3 mL of liquid remains in the round bottom flask to stop the reaction, pour the remaining liquid into an evaporating dish, and evaporate in a water bath (temperature 45 ℃) in a fume hood , evaporated until it was mucus, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com