In-vitro individualized medicine test method for lung cancer and culture medium
A culture medium and separation culture technology, applied in the field of cell biology and pharmacology, can solve the problems affecting the quality of life of patients, reduce the burden of toxicity and side effects, improve efficiency, and prolong survival time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
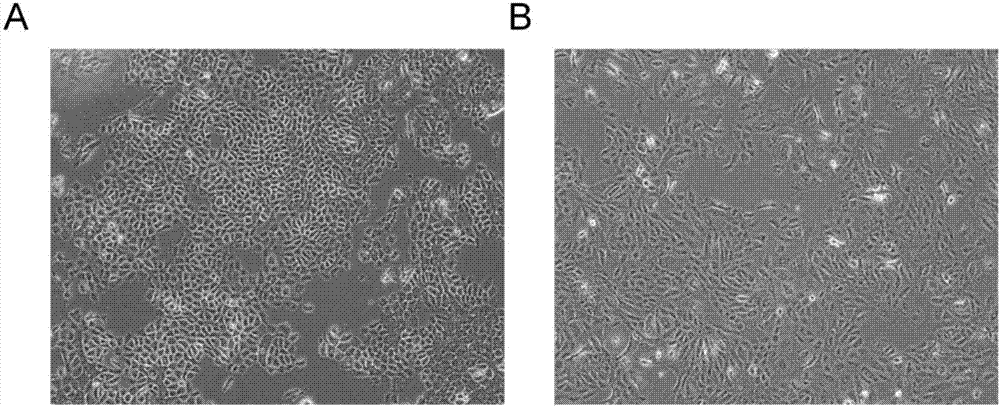
[0053] [Example 1] Medium M for separation, culture, and subculture of human or mammalian primary epithelial cells and primary tumor cells
[0054] Its composition includes: DMEM and Ham's F-12 NUTRIENT MIX mixed medium at a volume ratio of 3:1, while adding 5% fetal bovine serum, 2nM triiodothyronine, 0.5% insulin-transferrin-selenium reagent, 10 uM Y-27632, 10ng / mL epidermal growth factor, 0.4μg / mL hydrocortisone, 1.0nM cholera toxin, 0.5μg / mL amphotericin B, 40μg / mL gentamicin, Activin A 10 ng / ml and 3μg / ml recombinant human R-Spondin-1.
Embodiment 2
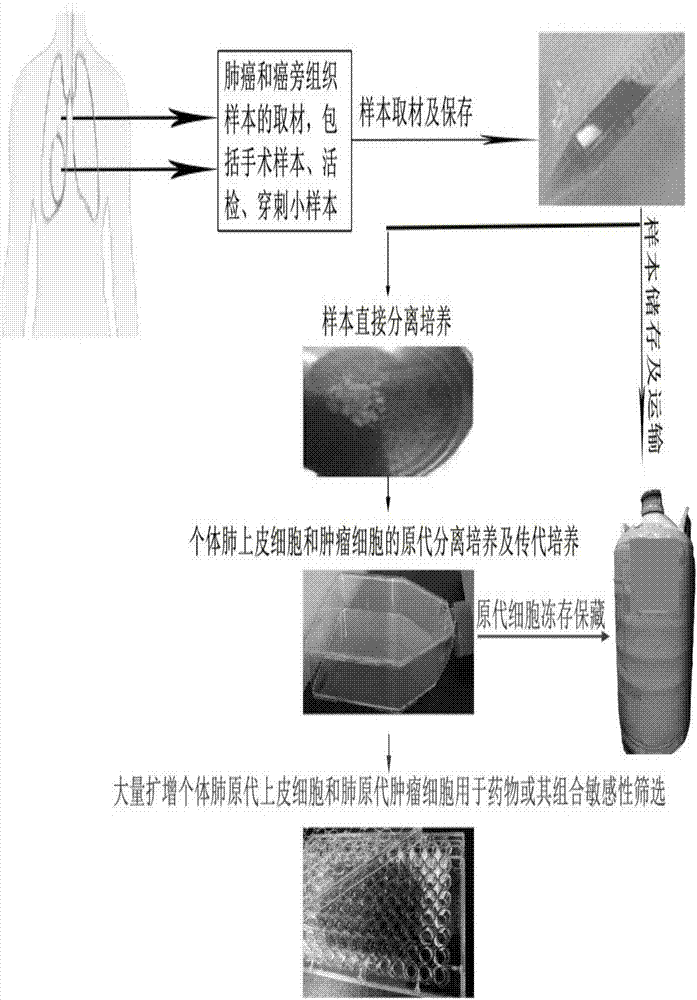
[0055] [Embodiment 2] Establishing the operation method of sampling of lung cancer and adjacent tissue samples and its storage and transportation
[0056] 1) With the informed consent of the patient and the patient’s guardian, the lung cancer and adjacent tissue samples used in this experiment are both about 1 cm 3 (Cubic centimeters), taken from a hospital surgical resection sample, the clinical stage of this patient is poorly differentiated lung adenocarcinoma, no other treatment.
[0057] 2) Under clean room conditions, in strict accordance with the standard of aseptic operation, the surgical samples are collected and stored in test tubes, and the sample collection fluid is added according to the sample size, generally 3-5mL, the sample collection fluid contains 2% penicillin / streptomycin And 100ug / ml nystatin PBS, because the sample size is still sufficient, it is divided into two at the same time to try direct isolation culture and long-term liquid nitrogen storage.
[0058] 3) ...
Embodiment 3
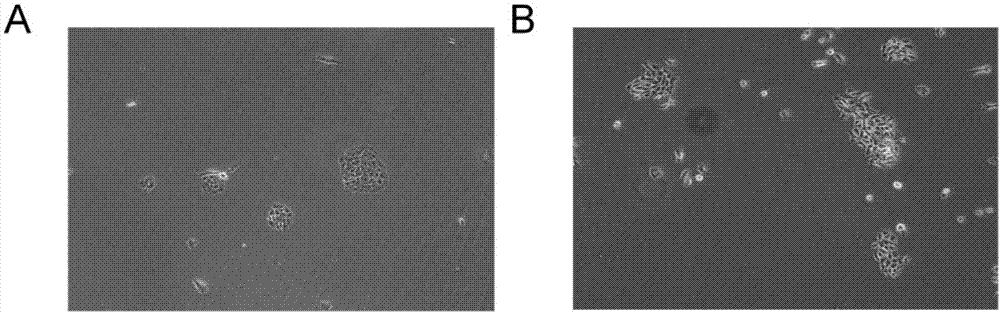
[0060] [Example 3] A method for establishing the primary isolation and culture of lung epithelial cells and tumor cells of lung cancer patients with rapid proliferation in a short period of time includes the following specific steps:
[0061] 1) Before the sample is directly separated and cultivated, the ultra-clean workbench is turned on and the UV lamp is turned on. After 30 minutes of work, turn off the UV lamp, turn on the fan to adjust the air volume, and ensure that the wind speed in the working area is always in the ideal state. Start experiment
[0062] 2) Prepare 5 100mm sterile cell culture dishes marked as ①②③④⑤ and place them in order. Add 10 mL of absolute ethanol (analytical purity, flammable, and keep away from the source of ignition) to the petri dish ②, and the petri dishes ③ and ④ respectively Add 10 mL of PBS buffer in an ice bath.
[0063] 3) Preparation of digestion solution: the above medium M containing 0.2mg / mL collagenase / hyaluronidase (07912, STEMCELL) (mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com