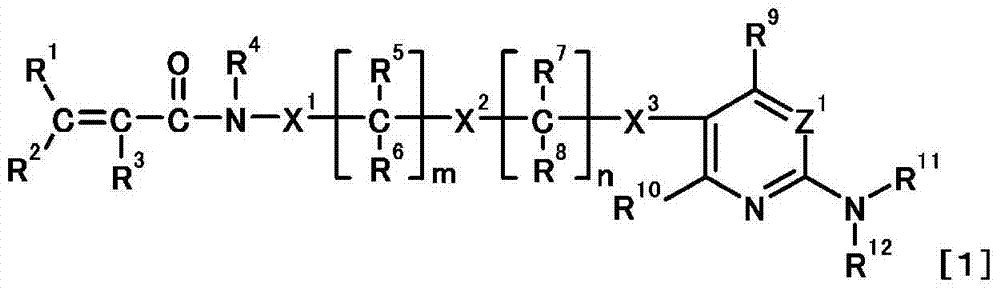
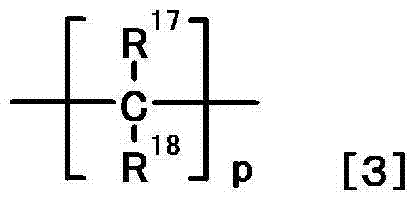Nitrogen-containing heterocyclic compound or salt thereof
A compound and heterocyclic technology, applied in the direction of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0844] (1)
[0845] [chemical formula 45]
[0846]
[0847] To a solution of 11.6 g of ethyl 4-chloro-2-(methylthio)pyrimidine-5-carboxylate in 100 mL of tetrahydrofuran, 8.4 mL of triethylamine and 5.1 mL of propylamine were added under ice-cooling, and stirred at room temperature for 30 minute. A 1.0 mol / L aqueous hydrochloric acid solution and ethyl acetate were added to the reaction mixture. The organic layer was separated, washed successively with saturated aqueous sodium bicarbonate and saturated aqueous sodium chloride, dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure to obtain oily 2-(methylthio)-4-( Propylamino)pyrimidine-5-carboxylic acid ethyl ester (A1) 11.7g.
[0848] 1 H-NMR (CDCl 3 )δ: 8.61 (1H, s), 8.27 (1H, brs), 4.32 (2H, q, J=7.0Hz), 3.55-3.48 (2H, m), 2.53 (3H, s), 1.73-1.60 (2H , m), 1.37(3H, t, J=7.3Hz), 0.99(3H, t, J=7.6Hz).
[0849] (2)
[0850] [chemical formula 46]
[0851]
[0852] In a s...
Embodiment 2
[0884] (1)
[0885] [chemical formula 53]
[0886]
[0887] 302 mg of N-(3-aminophenyl)-2,2,2-trifluoro-N-methylacetamide, 4-(propylamino)-2-((2- (Pyridin-4-yl) ethyl) amino) pyrimidine-5-carboxylic acid (A3) 627mg, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride 545mg and 1 Add 15 mL of N,N-dimethylformamide and 766 μL of triethylamine to 377 mg of -hydroxybenzotriazole monohydrate at room temperature, seal the reaction vessel, and stir at 100°C for 40 minutes using a microwave reactor . After cooling the reaction mixture to room temperature, it was added to saturated aqueous sodium bicarbonate and ethyl acetate. The organic layer was separated, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure. The resulting residue was purified by silica gel column chromatography (eluent: 97-96% ethyl acetate / methanol) to give 4-(propylamino)-2-((2-(pyridin-4-yl )ethyl)amino)-N-(3-(2,2,2-trifluoro-N-methylacetamide)phenyl)pyri...
Embodiment 3
[0907] Compounds (1-3) to (1-12) were obtained by the same method as in Example 1(7) or Example 1(8).
[0908] Table 16
[0909]
[0910] Table 17
[0911]
[0912] Table 18
[0913]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com