Method for determining unstable iron content in iron and carbohydrate complex
A technology of carbohydrates and carbohydrates, applied in the measurement of color/spectral characteristics, material analysis by observing the influence of chemical indicators, and analysis by making materials undergo chemical reactions, etc., can solve the problem of unstable iron content methods and other problems to achieve high accuracy, high detection sensitivity, and good precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of iron stock solution (100μg / ml): accurately weigh crystalline ferric ammonium sulfate [FeNH 4 (SO 4 ) 2 〃12H 2 O] (Aladdin Industrial Corporation) 0.860g, dissolve in deionized water, add 2.0ml of concentrated sulfuric acid (AR), transfer to a 1L volumetric flask and dilute to the mark with deionized water as a stock solution.
[0044] Preparation of iron standard series solution: Pipette 5, 10, 20, 30, 40ml of iron stock solution respectively, add them to a 100ml volumetric flask, add deionized water to the mark, shake up as the iron standard series solution, the concentrations are respectively 5, 10, 20, 30, 40μg / ml.
[0045] The iron standard curve is established: at room temperature, add 100μl each of the iron standard series solution into a 1.5ml EP tube, then add 700μl of an aqueous solution containing 200mM citric acid (reagent I), and finally add an aqueous solution containing 150mM ascorbic acid and 6mM ferroazine ( Reagent II) 350μL, after 60min react...
Embodiment 2
[0052] Establishment of iron standard curve: same as in Example 1.
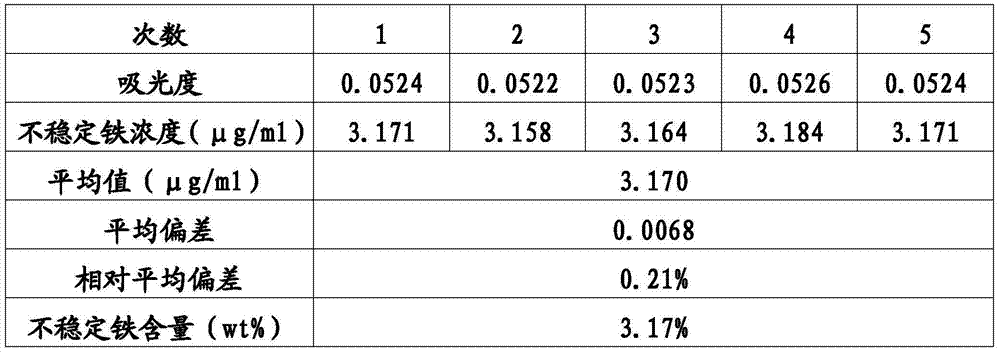
[0053] Detection method of unstable iron content in sodium iron gluconate preparation: similar to Example 1, except that when human serum is incubated with sodium iron gluconate sucrose complex injection, the amount of iron added is converted to a serum iron concentration of 100.0μg / mL. Take the same batch of sodium ferric gluconate preparation samples and repeat the experiment process 5 times for measurement and data calculation. The results are shown in Table 2.
[0054] Table 2
[0055]
Embodiment 3
[0057] Establishment of iron standard curve: similar to Example 1, except that sorbitol was used instead of ascorbic acid in reagent II.
[0058] Detection method of unstable iron content in sodium iron gluconate preparation: similar to Example 1, except that when human serum is incubated with sodium iron gluconate sucrose complex injection, the amount of iron added is converted to a serum iron concentration of 40.0μg / mL, CHAPS (Shanghai Haoyang Biotechnology Co., Ltd.) was used instead of thiourea in reagent I, and sorbitol was used instead of ascorbic acid in reagent II. Take the same batch of sodium ferric gluconate preparation samples and repeat the experiment process 5 times for measurement and data calculation. The results are shown in Table 3.
[0059] table 3
[0060]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com