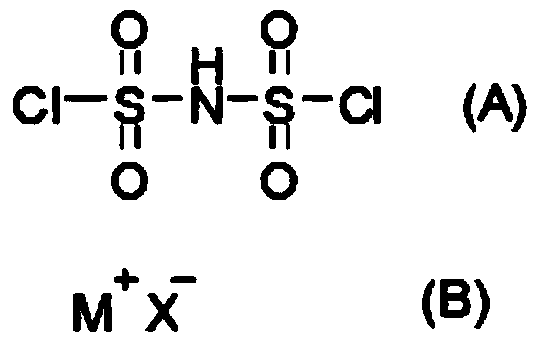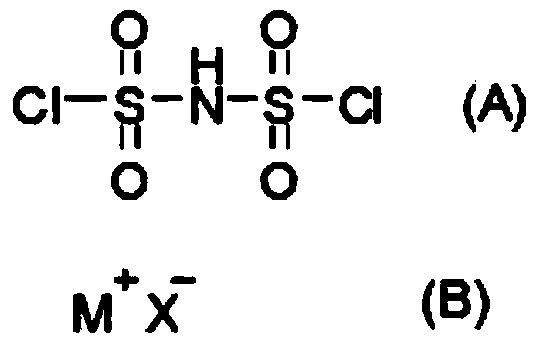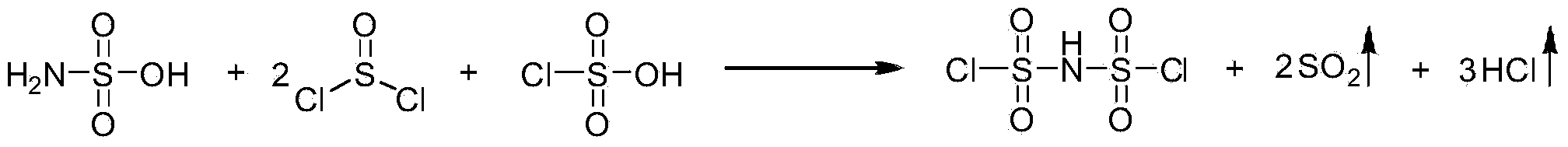Preparation method of bifluorosulfonyl imide onium salt
A technology of bisfluorosulfonimide onium salt and bisfluorosulfonimide, which is applied in the field of preparation of fluorine-containing compounds, can solve the problems that hinder the large-scale application of bisfluorosulfonimide onium salt, impurity ions and onium ions. Separation, preparation and purification process are long and other problems, to achieve the effect of large-scale industrial production, reduce material consumption, and reduce waste generation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] Concretely, the preparation method of the bisfluorosulfonimide onium salt provided by the present invention comprises reacting the bischlorosulfonimide represented by the structural formula (A), the onium ion halide represented by the structural formula (B) and hydrogen fluoride to prepare The step of obtaining bisfluorosulfonylimide salt.
[0018]
[0019] Among them, M + represents the onium cation, X - Indicates a halide ion.
[0020] In the above preparation method, the bischlorosulfonimide can be a commercially available product, or can be obtained by a method recorded in literature (R.Appel et al, Chem.Ber.1962,95,625; M.Goehring et al, Inorg. Synth.1966,8,105; J.Ruff,Inorg.Chem.1967,6,2108; M.Berran et al, Z.Anorg.Allg.Chem.2005,631,55) synthesized. Preferably, the dichlorosulfonimide is prepared by the mixed reaction of sulfonamide, thionyl chloride and chlorosulfonic acid, and the reaction equation is as follows:
[0021]
[0022] Wherein, the reactio...
Embodiment 1
[0031] Embodiment 1: Preparation of bisfluorosulfonimide
[0032]Under stirring, 679 g of sulfonamide (7 mol), 1785 g of thionyl chloride (15 mol), and 815.5 g of chlorosulfonic acid (7 mol) were successively added into a dry 5 L reaction vessel to obtain a mixed solution. Heat the mixed solution to 140°C for reaction, and the sulfur dioxide and hydrogen chloride acid gas produced will be absorbed by the lye. After reacting for 20 hours, the brownish-yellow liquid crude product obtained was vacuum-distilled under reduced pressure, and the fraction at 110-114°C / 2mmHg was collected to obtain 1438 g of dichlorosulfonimide colorless liquid, with a yield of 96% and a purity of 99%.
[0033] Embodiment 1: Preparation of bisfluorosulfonimide ammonium salt
[0034] Under constant temperature stirring at 5°C, add 1,600 grams of hydrogen fluoride (80 mol) into a 3L dry reaction vessel, and slowly add 1,284 grams of bischlorosulfonimide (6 mol) for a chemical reaction, and the generated...
Embodiment 2
[0035] Example 2: Preparation of bisfluorosulfonimide tetraethylammonium salt
[0036] Under constant temperature stirring at 8°C, add 500 grams of hydrogen fluoride (25 mol) into a 1L dry reaction vessel, slowly add 165.5 grams of tetraethylammonium chloride (1 mol) and 214 grams of dichlorosulfonimide (1 mol) for a chemical reaction to produce The hydrogen chloride acid gas is absorbed by the lye. The reaction was continued at a constant temperature of 8°C for 5 hours, and then heated to 50°C to distill and recover 450 g of hydrogen fluoride, and continue to heat and dry in vacuum to obtain 307 g of bisfluorosulfonimide tetraethylammonium salt with a yield of 99% and a purity of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com