New method for preparing L-tertiary leucine
A technology of tert-leucine and a new method, which is applied in the field of preparing L-tert-leucine, can solve problems such as high operation requirements, hidden safety hazards, and environmental pollution, and achieve the effects of reducing environmental pollution, improving safety, and being environmentally friendly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
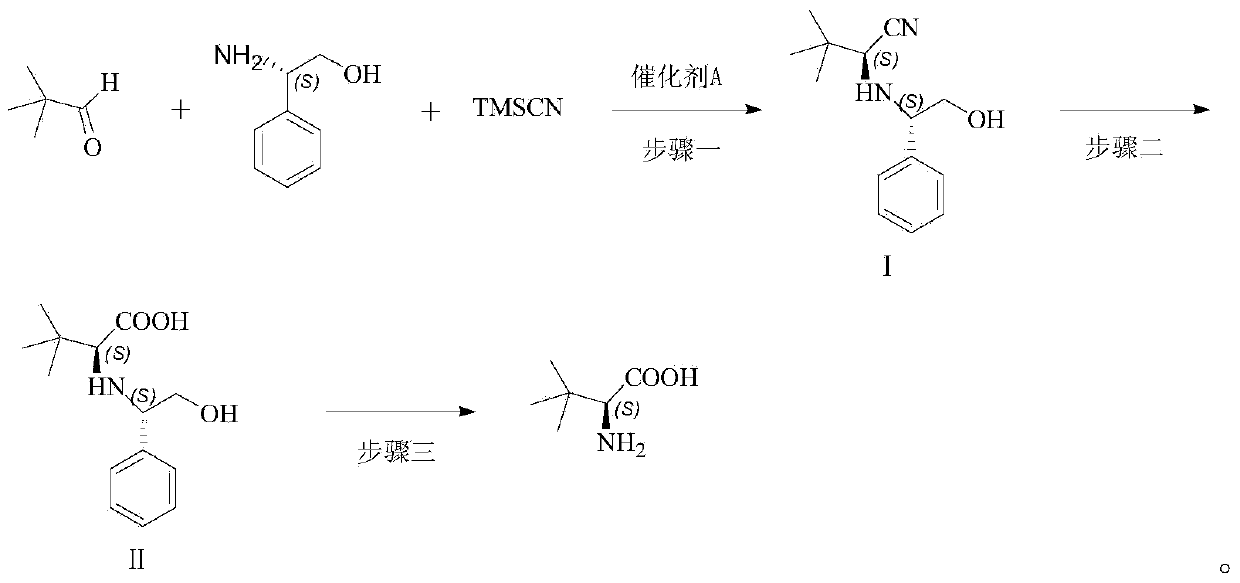
[0042] The synthesis of the aminonitrile compound of embodiment 1 magnesium diiodide catalysis
[0043] Pivalaldehyde (8.6g, 0.1mol) and L-phenylglycol (15.1g, 0.11mol) were added to a dry 100mL single-necked flask, and MgI was added under the protection of nitrogen. 2 (10mmol), after stirring for 10min, add TMSCN (11.9g, 0.12mol), stir the reaction at room temperature, TLC detects the reaction process, and adds Na after the end of the reaction. 2 S 2 o 3 The reaction was quenched with aqueous solution, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated and separated by column chromatography to obtain an aminonitrile compound (20.88 g, 0.09 mol) with a yield of 90%.
Embodiment 2
[0044] Embodiment 2 Synthesis of the aminonitrile compound catalyzed by magnesium dibromide
[0045] Add pivalaldehyde (10.32g, 0.12mol) and L-phenylglycol (18.08g, 0.132mol) into a dry 100mL single-necked flask, under the protection of nitrogen, add magnesium dibromide (12mmol), and stir for 10min Then add TMSCN (14.286g, 0.144mol), stir the reaction at room temperature, TLC detects the reaction progress, after the reaction finishes, add Na 2 S 2 o 3 The reaction was quenched with aqueous solution, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated and separated by column chromatography to obtain aminonitrile compound (24.22 g, 0.104 mol) with a yield of 87%.
Embodiment 3
[0046] Example 3 Synthesis of aminonitrile compounds catalyzed by magnesium dichloride
[0047]Pivalaldehyde (12.9g, 0.15mol) and L-phenylene glycol (22.6g, 0.165mol) were added to a dry 100mL single-necked flask, under the protection of nitrogen, magnesium dichloride (1.43g, 15mmol) was added, and stirred After 10min, TMSCN (17.8g, 0.18mol) was added, the reaction was stirred at room temperature, the reaction progress was detected by TLC, and Na 2 S 2 o 3 The reaction was quenched with aqueous solution, extracted with ethyl acetate, washed with saturated brine, dried over anhydrous sodium sulfate, concentrated and separated by column chromatography to obtain an aminonitrile compound (28.54 g, 0.12 mol) with a yield of 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com