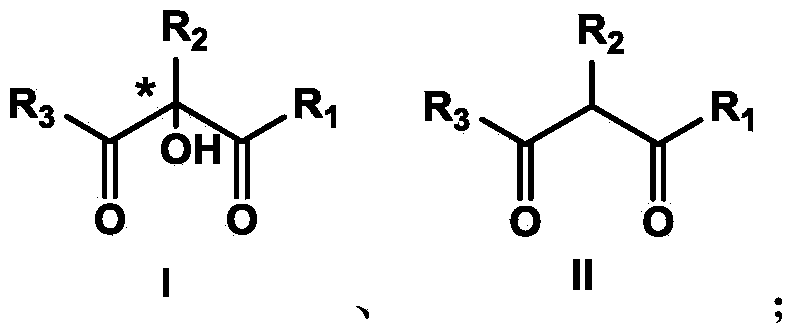
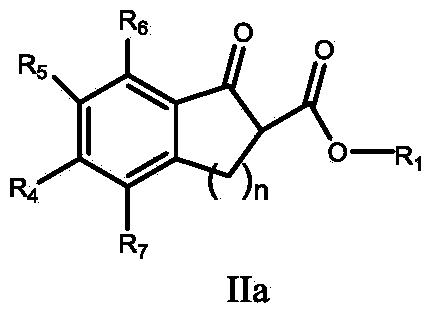
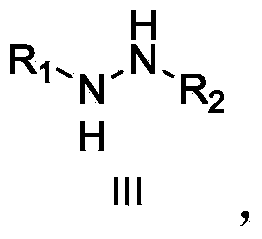
Method for preparing alpha-hydroxyl-beta-dicarbonyl compound through activating oxygen in air by using hydrazine
A technology for dicarbonyl compounds and compounds, applied in the preparation of organic compounds, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of harsh requirements and low catalytic activity, and achieve the effect of mild reaction conditions and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of 5-chloro-2-hydroxyl-1-indanone-2-carboxylic acid methyl ester
[0049] Weigh the substrate (22.4mg, 0.1mmol), cesium carbonate (32.5mg, 0.1mmol) and methylhydrazine (12.0mg, 0.26mmol), add 4mL of chloroform, and stir the reaction in air at room temperature. During the reaction, the conversion of the substrate was observed by TLC (thin layer chromatography). After reacting for 12 hours, the solvent was spin-dried and prepared by medium pressure (silica gel column, PE / EtOAc=5 / 1) to obtain the target product as a white solid (21.0 mg, 88% yield).
Embodiment 2
[0050] Example 2 Preparation of 5-chloro-2-hydroxyl-1-indanone-2-carboxylic acid methyl ester
[0051] Weigh the substrate (22.4mg, 0.1mmol), tetramethylguanidine (11.5mg, 0.1mmol) and methylhydrazine (12.0mg, 0.26mmol), add 4mL of chloroform, and stir the reaction in air at room temperature. During the reaction, the conversion of the substrate was observed by TLC (thin layer chromatography). After reacting for 6 hours, the solvent was spin-dried and prepared by medium pressure (silica gel column, PE / EtOAc=5 / 1) to obtain the target product as a white solid (20.3 mg, 88% yield).
Embodiment 3
[0052] Example 3 Preparation of (S)-5-chloro-2-hydroxyl-1-indanone-2-carboxylic acid methyl ester
[0053] Weigh the substrate (22.4mg, 0.1mmol), cinchonine (11.7mg, 0.04mmol) and methylhydrazine (6.0mg, 0.13mmol) into a reaction flask, and add 4mL of chloroform. The reaction temperature was controlled at 15°C, and the reaction was stirred in the open air. During the reaction, the conversion of the substrate was observed by TLC (thin layer chromatography). After 24 hours of reaction, the solvent was spin-dried and prepared by medium pressure (silica gel column, PE / EtOAc=5 / 1) to obtain the target product as a white solid (19.4 mg, 81% yield, 51% ee).
[0054] Example 3: Preparation of (S)-5-chloro-2-hydroxyl-1-indanone-2-carboxylic acid methyl ester
[0055] Weigh the substrate (22.4mg, 0.1mmol), dihydrocinchonine (11.7mg, 0.04mmol) and methylhydrazine (6.0mg, 0.13mmol) into a reaction flask, and add 4mL of chloroform. The reaction temperature was controlled at 15°C, and the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com