A tumor necrosis factor-related apoptosis ligand fusion protein and its preparation method and application
A tumor necrosis factor and fusion protein technology, applied in the field of tumor necrosis factor-related apoptosis ligand fusion protein, can solve the problems of TRAIL instability, increased sensitivity, short half-life, etc., achieve good pharmacokinetic effect and prolong half-life Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Design, expression, preparation and identification of tumor necrosis factor-related apoptosis ligand fusion protein
[0092] Using computer-aided structural simulation and molecular design, on the SGI computer workstation, using MSI's molecular design software (InsightII, Discover and other modules), based on the crystal structure of tumor necrosis factor-related apoptosis ligand and annexin V, Molecular modeling and molecular design were carried out for tumor necrosis factor-related apoptosis ligand and annexin V fusion protein, and the amino acid length of the connecting peptide was determined.
[0093]In the computer-aided molecular design of the tumor necrosis factor-related apoptosis ligand fusion protein, the inventors carried out structural simulation and molecular design on the tumor necrosis factor-related apoptosis ligand fusion protein, in the tumor necrosis factor-related apoptosis ligand and Based on the crystal structure of annexin V, molecular modeling an...
Embodiment 2
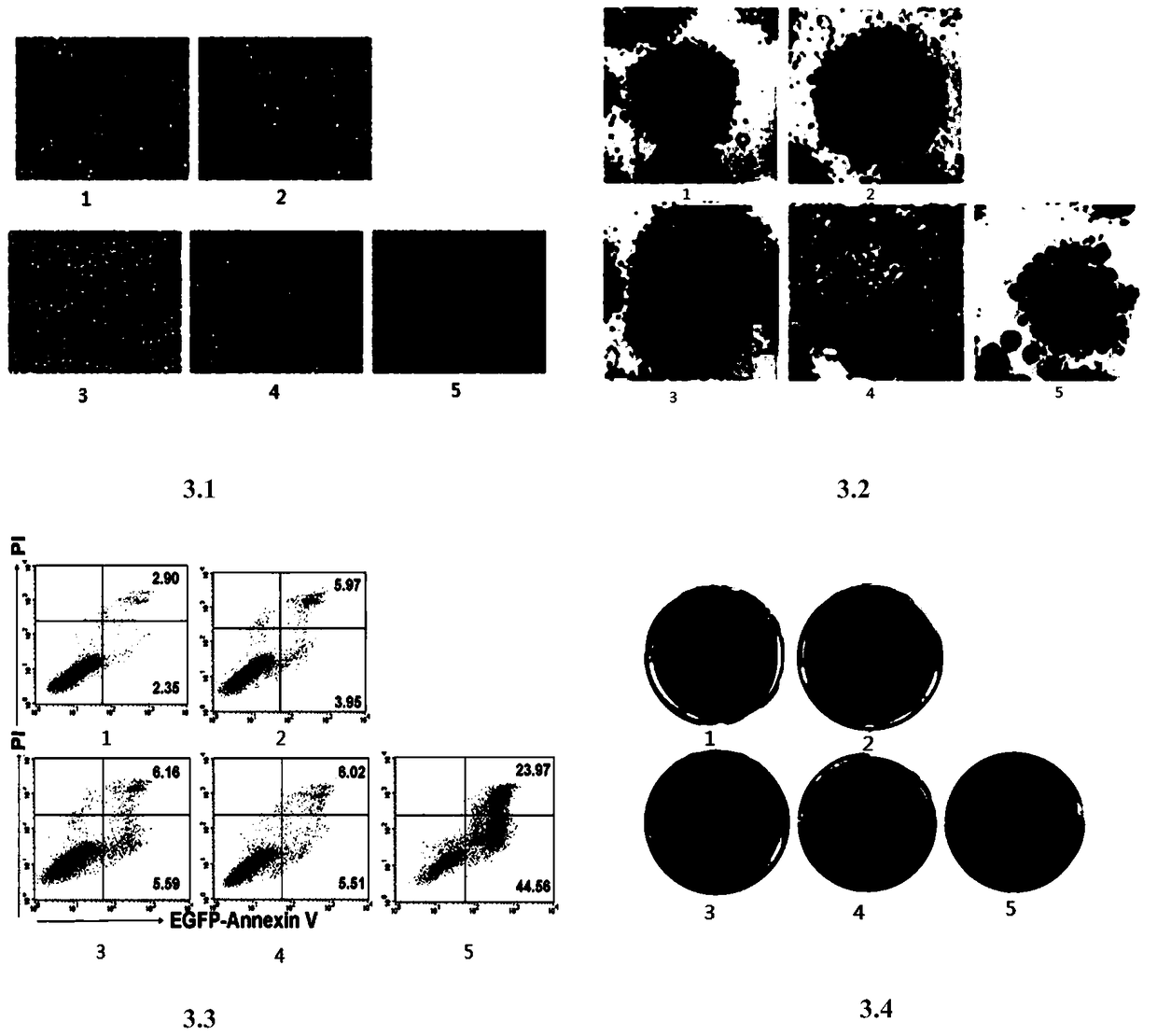
[0102] Detection of Sensitivity of Different Human Tumor Cell Lines to Tumor Necrosis Factor-related Apoptosis Ligand Fusion Protein TP8
[0103]Different human tumor cell lines: colon cancer Colo-205, breast cancer MDA-MB-231, cervical cancer Hela, small cell lung cancer H446, liver cancer PLC, non-small cell lung cancer H1229, liver cancer Bel7402, non-small cell lung cancer A549, Breast cancer MCF-7, lymphoma U937, liver cancer HepG2, colon cancer HT29, breast cancer highly metastatic cell MDA-MB-435, tongue squamous cell carcinoma TCA8113, colon cancer HCT116, pancreatic cancer SW1990, K562 cells, chronic myelogenous leukemia K562, etc. In DMEM containing 10% newborn bovine serum (containing 100 U / ml penicillin and 100 μg / ml streptomycin), and placed in an incubator at 37°C and 5% CO2. K562 and Colo205 were grown in RPMI1640 (containing 100 U / ml penicillin and 100 μg / ml streptomycin) containing 10% newborn bovine serum in an incubator at 37°C and 5% CO2. All cells were pu...
Embodiment 3
[0115] Mechanism Analysis of Tumor Necrosis Factor-related Apoptosis Ligand Fusion Protein
[0116] Western Blot detects the expression of important proteins related to the apoptotic pathway from the protein level: A549 cells are placed in a 6-well cell culture plate to grow to a density of 80%, and treated with an equimolar amount of drugs as required, and after continuing to culture for 6 hours, Collect the cells, wash the cell pellet once with pre-cooled PBS, add an appropriate amount of cell lysate (containing protease inhibitors, Roche Company) to suspend and mix well, place on ice for 30 minutes, centrifuge at 12,000 rpm for 15 minutes at 4°C, and pipette Clear, use the Coomassie brilliant blue method (Bradford method) to measure the protein concentration, adjust the protein concentration, add 4×loading buffer and boil the sample in a boiling water bath for 5 minutes, and store the sample at -20°C for later use.
[0117] Prepare 12% SDS-PAGE gel according to the formula ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com