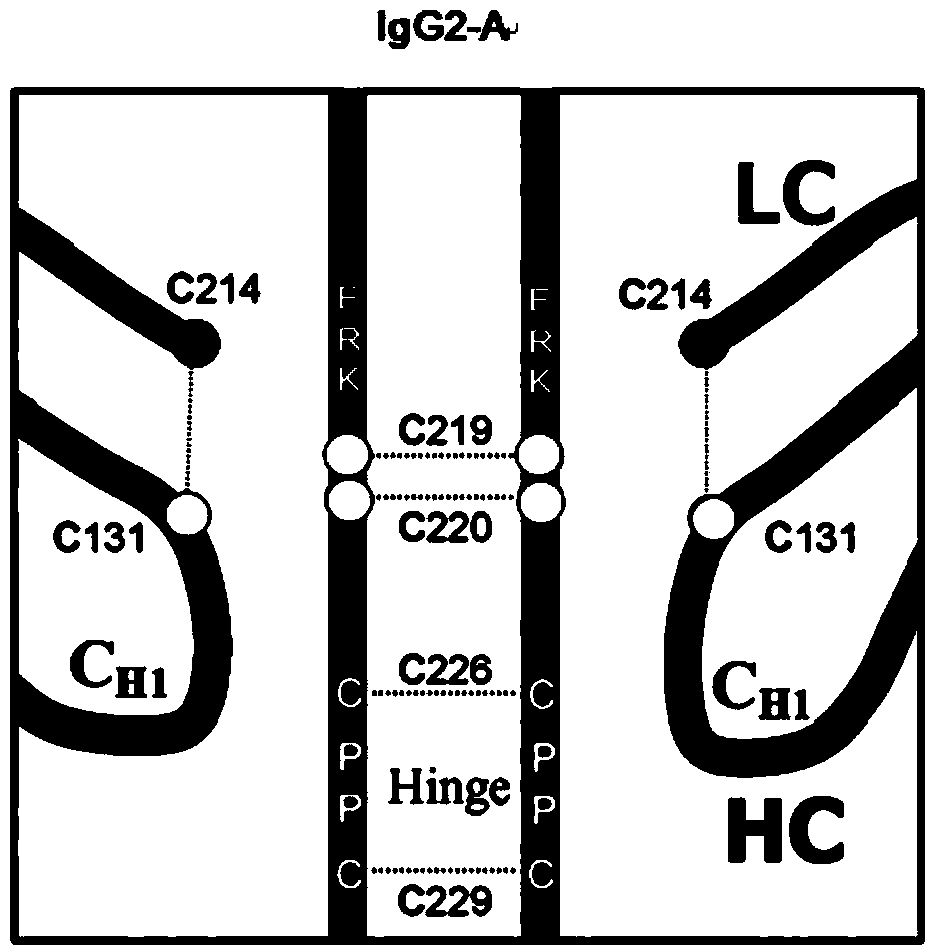
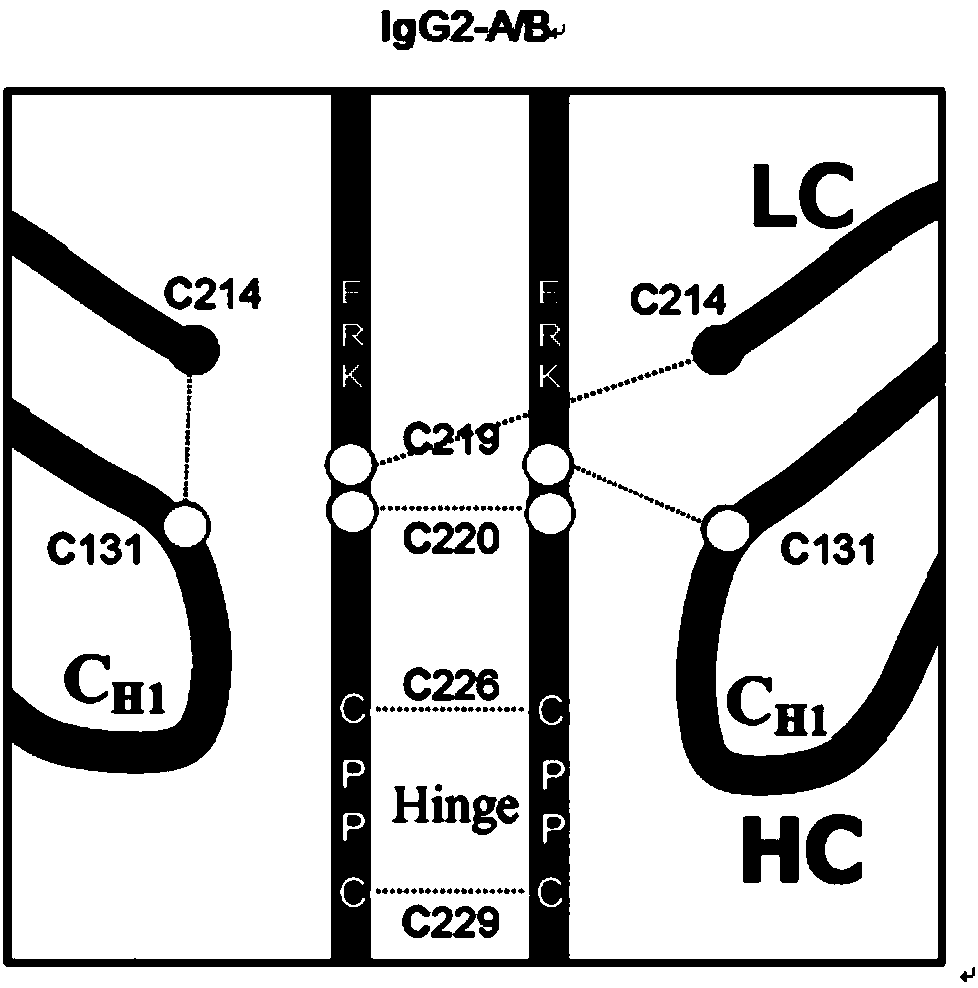
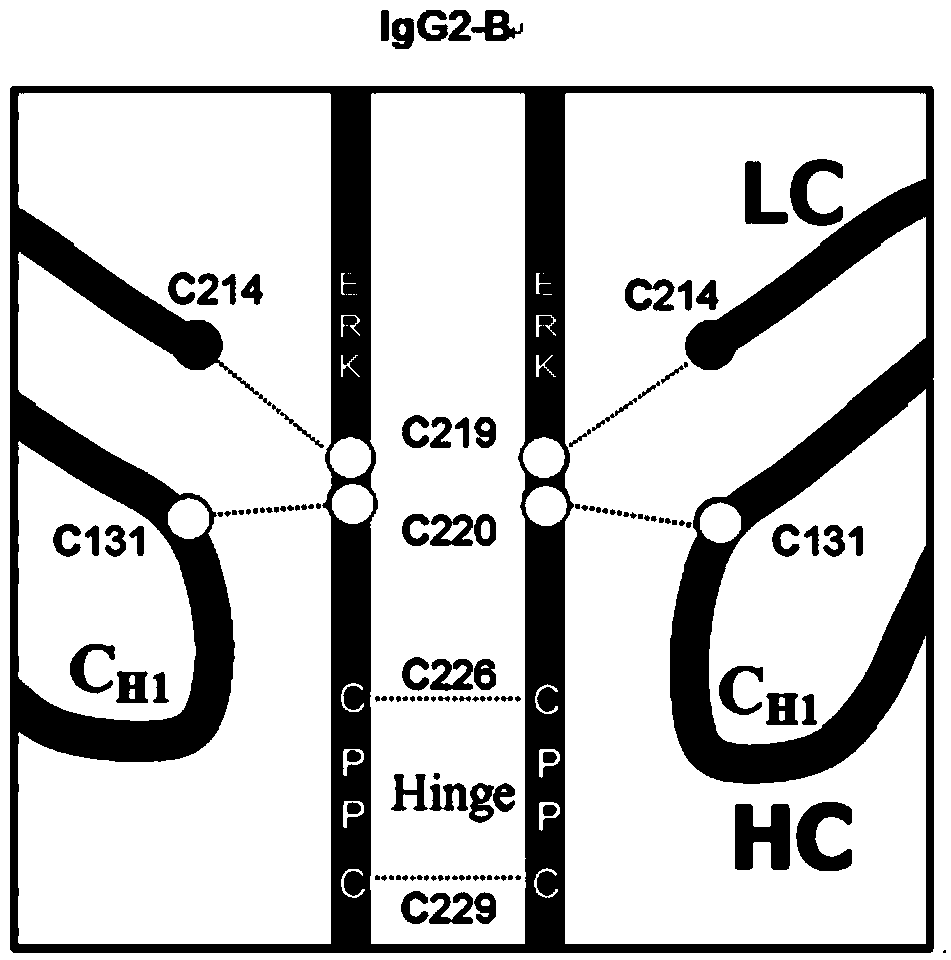
Hinge region modification body of human IgG2 antibody
A hinge region and antibody technology, applied in the direction of antibodies, cells modified by introducing foreign genetic material, anti-animal/human immunoglobulin, etc., can solve the problems of increasing the sensitivity and reducing stability of IgG2 antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, wild-type Denosumab and AB4 and antigen-binding affinity determination
[0046] Firstly, the binding ability of HRP-labeled Denosumab antibody to antigen was detected by direct ELISA method. First, horseradish peroxidase (HRP, Boehringer Ingelheim)-labeled anti-human RANKL antibody Denosumab (Amgen) was used as a reagent (350 μg / mL), and serially diluted in proportion. The antigen RANKL-mFc (100 μl, 0.5 μg / mL) fused with mouse IgG Fc was coated on a microtiter plate (Corning) and left overnight at room temperature. The coating solution was discarded, each well was blocked with skimmed milk dissolved in PBS for 0.5 hour, and then washed with PBS containing 0.05% Tween-20. Then 50 μl of HRP-labeled antibody Denosumab at different concentrations was added to each well, and after reacting for 1 hour, the wells were washed with PBS containing 0.05% Tween-20. Finally, the OD value of each well was read with a microplate reader (Thermo Fisher) at dual wavelengt...
Embodiment 2
[0048] Example 2. Competitive binding assay with wild-type Denosumab antibody
[0049] HRP-labeled anti-human RANKL antibody Denosumab was used as a reagent (initial concentration 350 μg / mL, diluted 1:1000). Coat the microtiter plate with the antigen RANKL-mFc (100 μl, 0.5 μg / mL) fused with mouse IgG Fc and leave overnight at room temperature. The coating solution was discarded, each well was blocked with skimmed milk dissolved in PBS for 0.5 hour, and then washed with PBS containing 0.05% Tween Tween-20. Then, a mixture of 50 μl AB4 and 50 μl HRP-labeled antibody Denosumab (250ng / mL) was added to each well, and after reacting for 1 hour, the wells were washed with PBS containing 0.05% Tween-20 (Tween-20). Unlabeled Denosumab and an unrelated antibody against another antigen were used as positive and negative controls. The initial concentration of the test antibody and control antibody was 30 μg / mL, and then serially diluted at a ratio of 1:2. Finally, read the OD value of e...
Embodiment 3
[0050] Example 3, RP-HPLC analysis of heterogeneity between wild-type Denosumab antibody and AB4 antibody
[0051] RP-HPLC was used to analyze the heterogeneity caused by the disulfide bond between the AB4 antibody and the control antibody, that is, the wild-type Denosumab antibody. Wufeng HPLC LC100 high performance liquid chromatograph is used, equipped with P100 high pressure constant flow pump, UV100 ultraviolet detector and WS100 chromatographic workstation.
[0052] Molecular sieve HPLC chromatography conditions: gel chromatography column TSK gel G3000 SWXL (7.8mm×300mm) (TOSOH Company, Cat No. 0008541); mobile phase: 100mM sodium phosphate, 500mM sodium chloride, 5% ethanol, pH 7.0; flow rate: 0.5mL / min; column temperature: 25°C; injection volume: 20μl.
[0053] RP-HPLC conditions: Sephasil Peptide C8 chromatography column (4.6mm×250mm, 5μm, Pharmacia Biotech); mobile phase A: 0.1% trifluoroacetic acid aqueous solution; mobile phase B: 70% isopropanol, 20% acetonitrile...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com