Milrinone compound and pharmaceutical composition containing milrinone compound
The technology of a composition and milrinone is applied in the direction of drug combination, medical preparations containing active ingredients, drug delivery, etc., and can solve the effects of milrinone on drug efficacy and bioavailability, poor stability and clarity, and high impurity content, etc. problem, to achieve the effect of high safety, avoid side effects, and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
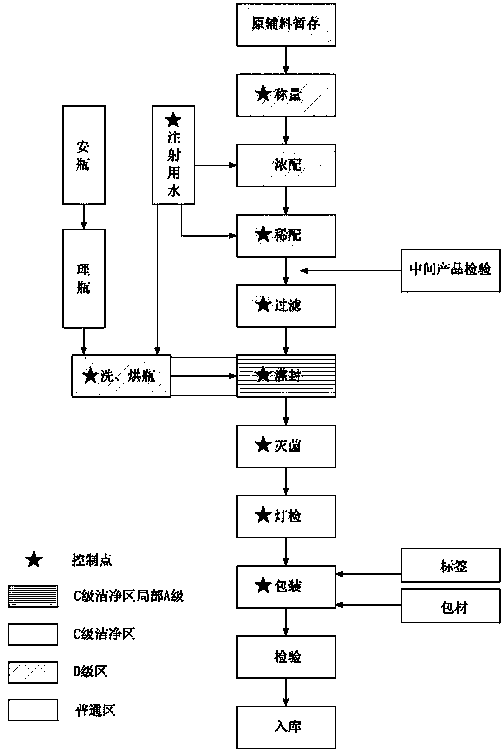
Image
Examples
Embodiment 1
[0031] Milrinone 1g
[0033] Lactic acid amount
[0034] Water for injection 1000ml
[0035] Preparation Process:
[0036] (1) Add lactic acid to 150 ml of water to adjust the pH to about 2.0, raise the temperature to 80°C while stirring, add 1g of milrinone while stirring after heating, and continue stirring for 10-20 minutes. Then add an appropriate amount of activated carbon with a concentration of 0.15%, and continue stirring for 10-15 minutes.
[0037] (2) Add 500ml of water to the above mixture to keep the temperature at 75-80°C, then add 9g of sodium chloride, and at the same time add an appropriate amount of 0.10% activated carbon, and continue stirring at this temperature for 10-15 minutes.
[0038] (3) Add water to the full amount, keep the temperature at 75-80°C, add an appropriate amount of 0.05% activated carbon at the same time, stir for 10-15 minutes, and adjust the pH at 3.0-4.3 with an appropriate amount of lactic ac...
Embodiment 2
[0042] Milrinone 2g
[0044] Appropriate amount of lactic acid
[0045] Water for injection 2000ml
[0046] Preparation Process:
[0047] (1) Add lactic acid to 300ml of water to adjust the pH to about 2.0, raise the temperature to 80°C with stirring, add 1g of milrinone with stirring after heating, and continue stirring for 10-20 minutes. Then add an appropriate amount of activated carbon with a concentration of 0.15%, and continue stirring for 10-15 minutes.
[0048] (2) Add 1000ml of water to the above mixture to keep the temperature at 75-80°C, then add 9g of sodium chloride, and at the same time add an appropriate amount of 0.10% activated carbon, and continue stirring at this temperature for 10-15 minutes.
[0049](3) Add water to the full amount, keep the temperature at 75-80°C, add an appropriate amount of 0.05% activated carbon at the same time, stir for 10-15 minutes, and adjust the pH at 3.0-4.3 with an appropriate amount of lactic a...
experiment example 1
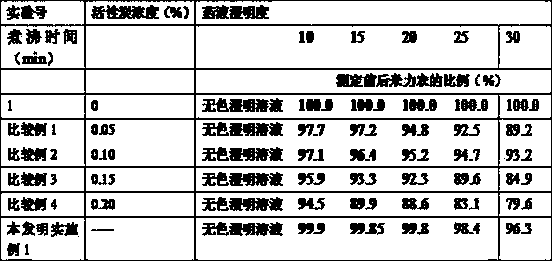
[0066] This experimental example is mainly to test the adsorption of activated carbon to milrinone.
[0067] In the preparation of injections, an appropriate amount of activated carbon with a concentration of 0.01%-0.5% (g / ml) is usually added to improve the clarity of the liquid and the effect of adsorbing pyrogens, and it may also have a certain adsorption effect on the main drug. In the patent CN102579329A, the verification experiment of the influence of different concentrations of activated carbon on the clarity and color of the liquid medicine and the content of milrinone was given, which proved that activated carbon has a certain adsorption on the main drug milrinone, and with the increase of the concentration of activated carbon The adsorption of milrinone is obvious. Therefore, in order to ensure the effect of activated carbon in removing pyrogens, it is generally recommended to use activated carbon with a concentration of 0.05% for 20-30 minutes during the preparation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com