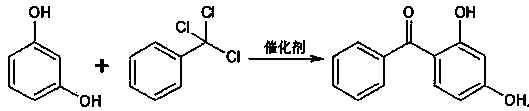
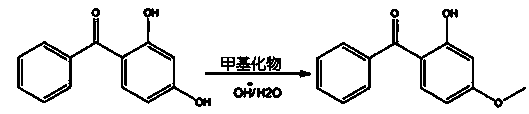
Synthetic method of 2-hydroxy-4-methoxybenzophenone
A technology of methoxybenzophenone and synthesis method, which is applied to the preparation of carbon-based compounds, chemical instruments and methods, and condensation to prepare carbonyl compounds, etc., which can solve problems such as low product yield, low product purity, and complex production process , to achieve the effect of simple reaction process, full and complete reaction, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Condensation reaction: Add 180ml of solvent methanol and 66g of resorcinol to a 500ml three-necked flask. After heating up and dissolving, add 6g of catalyst zinc chloride, then raise the temperature to 45°C, start adding 135g of trichlorotoluene dropwise, and control the temperature not to exceed 55°C. ℃, drop over 1h. After controlling the temperature at 55-60°C for 6 hours, raise the temperature to 70°C for 1 hour, cool to below 20°C, and filter. Wash with hot water at 50-60°C until neutral. Dry at 80°C for 6 hours, and the obtained product is 124g.
[0021] Methylation reaction: In a 250ml three-necked flask, add 8.2g of sodium hydroxide, dissolve in 100ml of water, and cool down to 5°C. Add 42.8g of 2,4-dihydroxybenzophenone, then lower the temperature to below 0°C, start to drop 30.6g of dimethyl sulfate, and control the dropping time for 1h. After the dropwise addition was completed, the temperature was controlled at 0-2°C and the reaction was stirred for more...
Embodiment 2
[0024] Condensation reaction: Add 170ml of solvent ethanol and 55g of resorcinol to a 500ml three-necked flask. After heating up and dissolving, add 2.7g of catalyst zinc chloride, then raise the temperature to 50°C, start to add 102.6g of trichlorotoluene dropwise, and control the temperature. More than 55 ℃, 1h dripping. After controlling the temperature at 55-70°C for 6 hours, raise the temperature to 75°C for 1 hour, cool to below 20°C, and filter. Wash with hot water at 50-60°C until neutral. Dry at 80°C for 6 hours, and the obtained product is 125g.
[0025] Methylation reaction: In a 250ml three-necked flask, add 4.1g of sodium hydroxide, dissolve in 54ml of water, and cool down to 5°C. Add 21.4g of 2,4-dihydroxybenzophenone, then lower the temperature to below 0°C, start to drop 10.8g of dimethyl carbonate, and control the dropping time for 1h. After the dropwise addition, control the temperature at 0-2°C and stir the reaction for more than 6 hours, remove the solve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com