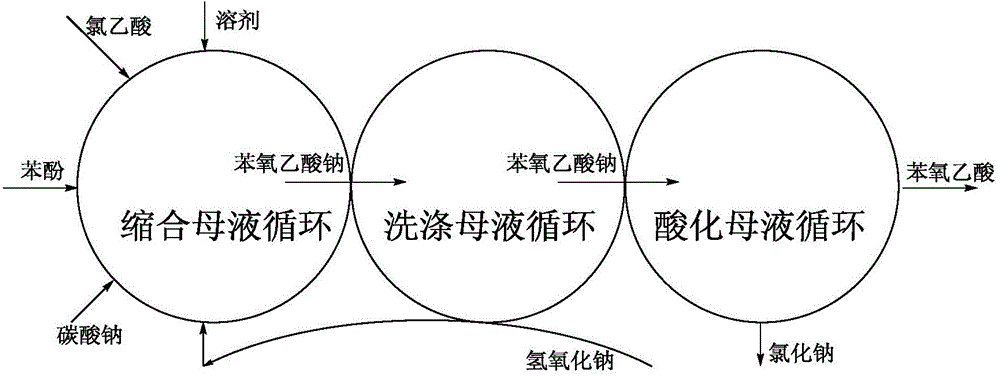
Ternary-cycle type no-waste-free preparing method for phenoxyacetic acid
A technology of phenoxyacetic acid and sodium phenoxyacetic acid, which is applied in the chemical industry, can solve the problems of waste water pollution and low yield, achieve high conversion rate and improve efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Add 38.4mL of water and 29.76g of chloroacetic acid into a 250mL single-necked flask and stir to dissolve, then add 16.7g of sodium carbonate solid to make the solution pH=6, and obtain a sodium chloroacetate solution;
[0046] Drop into 150mL chlorobenzene in 500mL there-necked flask, drop into 33.84g phenol, dropwise add 36g mass concentration while stirring and be the sodium hydroxide solution of 40%, make solution pH=12, obtain sodium phenoxide solution;
[0047] Heat and reflux the three-necked flask, keep the solution temperature at 91±1°C, add 0.1 g of catalyst (ferrous chloride-EDTA-sodium iodide), and then add the sodium chloroacetate solution obtained from the above reaction dropwise into the flask, approximately Add for 30 minutes, reflux for 1 hour after the dropwise addition, and keep the system pH=11.5~12; after the reaction, cool the flask to 40°C, add 5mL of concentrated hydrochloric acid with a mass concentration of 30% dropwise to make the system pH=7 ...
Embodiment 2
[0051] Add 51.2g of the condensed mother liquor water and 29.76g of chloroacetic acid separated in Example 1 into a 250mL single-necked flask, stir and dissolve, add 16.7g of sodium carbonate solid to the inside to make the solution pH=6, and obtain a sodium chloroacetate solution;
[0052] Get the remaining 28.8g of the condensation mother liquor water separated in Example 1, add 14.4g of solid sodium hydroxide to obtain a sodium hydroxide solution, which is used to regenerate and wash the mother liquor in the next cycle;
[0053] In the 500mL there-necked flask, drop into the condensation mother liquor that embodiment 1 separates and obtain, drop into 28.2g phenol, stir, then add the phenol extracting liquid caustic soda in embodiment 1, make pH=12 in the system, obtain sodium phenolate solution;
[0054] Add 0.014 g of ferrous chloride, the catalyst component, and add the sodium chloroacetate solution obtained above into the three-necked flask at one time. After the addition...
Embodiment 3
[0060] Add 59.5mL of water and 29.77g of chloroacetic acid into a 250mL single-necked flask and stir to dissolve, then add 16.70g of sodium carbonate solid to make the solution pH = 6, and obtain a sodium chloroacetate solution;
[0061] Drop into 200mL toluene in 500mL there-necked flask, drop into 35.25g phenol, dropwise add 46.88g mass concentration while stirring and be the sodium hydroxide solution of 32%, make solution pH=12, obtain sodium phenoxide solution;
[0062] Heat and reflux the three-necked flask, keep the solution temperature at 94±1°C, add 0.1 g of catalyst (ferrous chloride-EDTA-sodium iodide), and dropwise add the sodium chloroacetate solution obtained from the above reaction into the flask, about For 40 minutes, reflux for 2 hours after the dropwise addition, and keep the system pH=11-11.5; after the reaction, cool the flask to 30°C, add 6mL of concentrated hydrochloric acid with a mass concentration of 36% dropwise, to make the system pH=5; Suction filtra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com