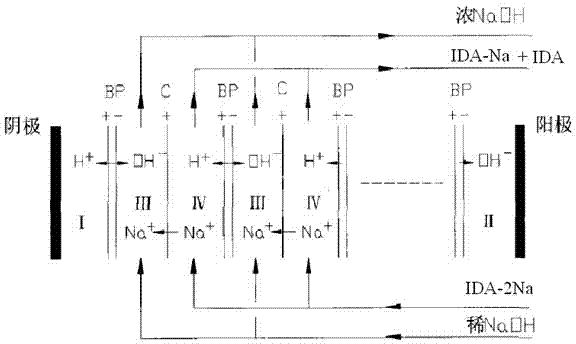
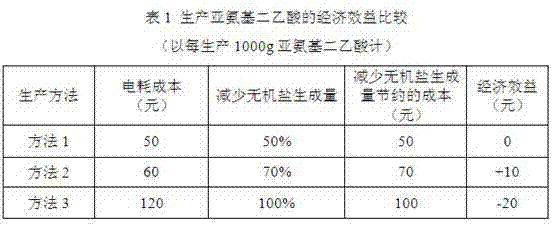
Iminodiacetic acid energy saving cleaning production method
A technology of iminodiacetic acid and iminodiacetic acid disalt is applied in the field of energy-saving and clean production of iminodiacetic acid, which can solve the problems of short conversion time in the early stage, increased demand for membranes, and difficulty in implementation, and reduce the generation of inorganic salts. The effect of saving sulfuric acid and steam, saving production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Prepare iminodiacetonitrile reaction solution: add aluminum trioxide to hydroxyacetonitrile with a concentration of 55% by weight, and the addition amount is 0.5% by weight of hydroxyacetonitrile, stir and mix well at room temperature, then acidify with dilute sulfuric acid to pH value Is 5, preheated to 50℃; preheated 25% by weight ammonia water to 170℃; respectively enter the continuous reactor with metering pump, the flow rate of hydroxyacetonitrile is 2m 3 / h, the reaction temperature is controlled at 120°C~130°C, the reaction pressure is 0.5Mpa, the residence time of the materials in the reactor is 0.5 minutes; the reaction mixture at the outlet of the reactor is extremely cold to 100°C to obtain the iminodiacetonitrile reaction liquid, According to analysis, the content of iminobisacetonitrile is 310g / L, the content of hydroxyacetonitrile is 30g / L, the content of triacetonitrile is 10g / L, the content of aminoacetonitrile is 1g / L, the content of ammonia is 5g / L, The ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com