A kind of engineering bacterium and the method for preparing (3r, 5r) 6-cyano-3,5-dihydroxyhexanoic acid tert-butyl ester
A technology of tert-butyl hydroxycaproate and engineering bacteria, applied in the field of biopharmaceuticals, can solve the problems of increased difficulty, increased difficulty of post-processing, increased production cost, etc., and achieve the effect of reducing production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
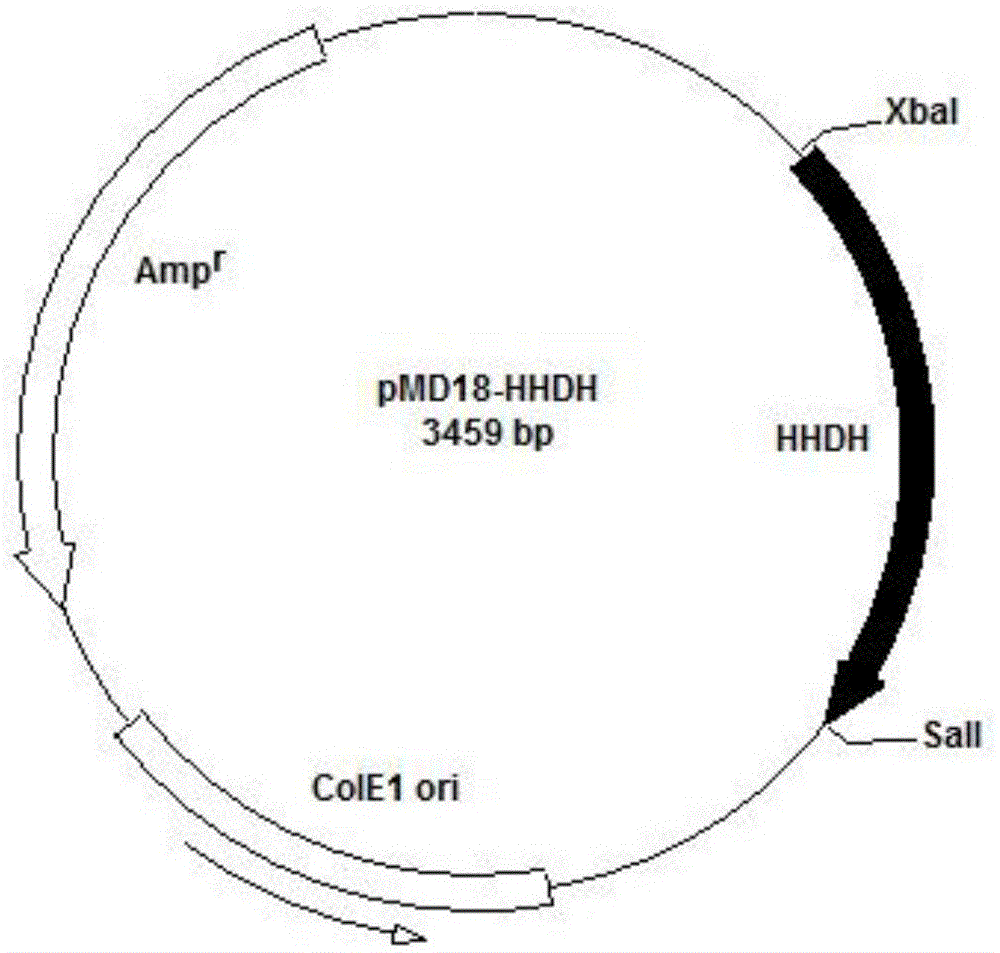
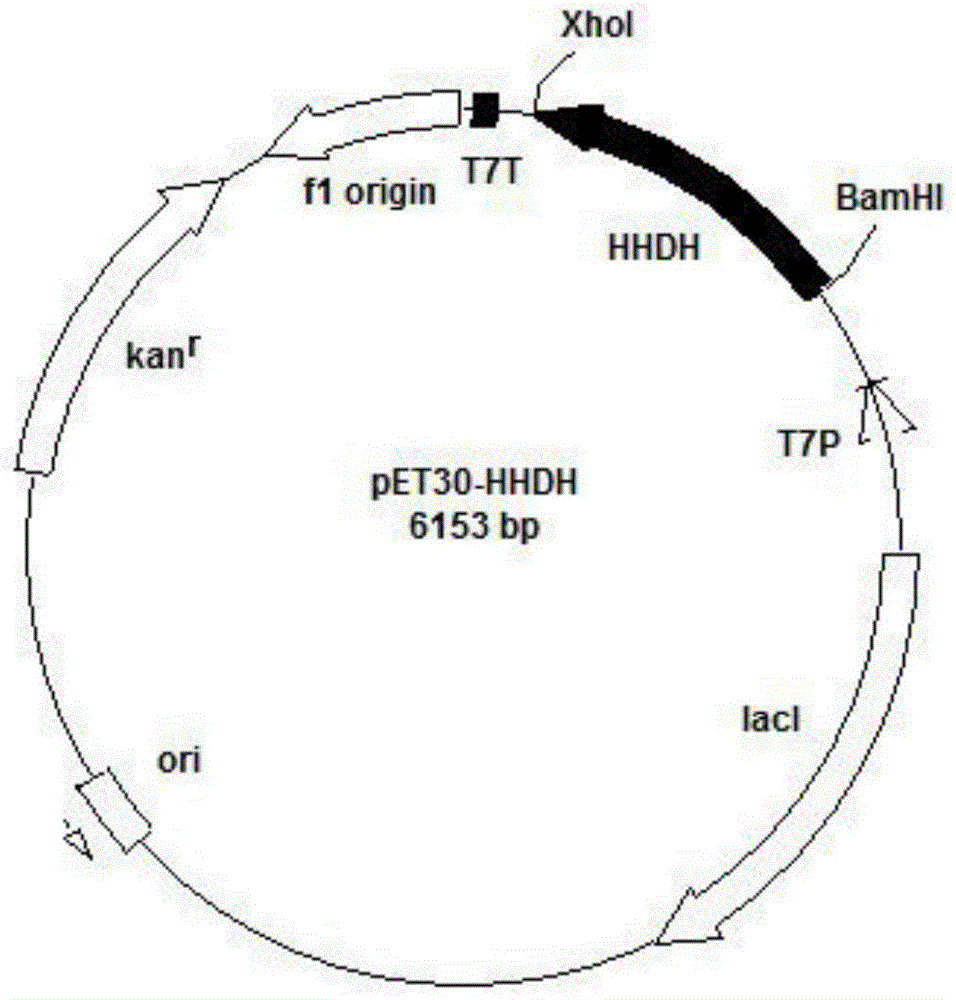
[0049] Construction of embodiment 1 plasmid pET30-HHDH
[0050] The hhdh gene was cloned with primers F_HHDH / R_HHDH to obtain a hhdh gene with a length of 765bp. Nucleic acid electrophoresis to verify gene size, such as figure 2 .
[0051] The sequence of primer F_HHDH is:
[0052] 5'-CGCGGATCCATGTCAACCGCAATTGTAAC-3';
[0053] The sequence of primer R_HHDH is:
[0054] 5'-CCGCTCGAGCTACTCTGGCATACCAGG-3'.
[0055] The hhdh gene sequence is as follows:
[0056] ATGTCAACCGCAATTGTAACAAACGTTAAGCATTTTGGGGGAATGGGGTCTGCACTTCGTCTCTCGGAAGCAGGACATACAGTGGCTTGCCACGATGAAAGCTTCAAACACCAAGACGAACTTGAAGCCTTTGCCGAAACCTATCCACAACTCATCCCAATGTCGGAACAAGAACCAGCGGAACTCATCGAGGCAGTTACCTCCGCTCTCGGTCACGTTGATGTACTTGTGAGCAACGACATCGCTCCGGTCGAGTGGCGCCCAATCGATAAATACGCTGTAGAGGACTATCGCGATACTGTCGAGGCGCTCCAAATTAAGCCATTTGCACTGGTCAACGCCGTTGCAAGTCAAATGAAGAAGCGCAAAAGCGGACATATTATCTTTATTACCTCTGCTGCTCCAGTTGGGCCTTGGAAGGAACTTTCTACCTACTCGTCAGCCCGTGCAGGTGCATCTGCTTTGGCAAATGCCCTTTCGAAGGAACTCGGTGAATACAACATTCCGGTGTTCGCAATCGCT...
Embodiment 2
[0058] Construction and induced expression of embodiment 2 genetically engineered bacteria
[0059] The plasmid pET30-HHDH constructed in Example 1 was used to transform the expression host EscherichiacoliBL21(DE3). Use primers F_HHDH / R_HHDH for colony PCR to verify transformed recombinants. The verified genetically engineered bacteria is EcoH. EcoH was inoculated into the fermentation medium and cultured with constant temperature shaking for 16 hours. The culture conditions were 35° C. and 180 rpm. When the cell concentration grows to OD 600 When =0.8, add 0.4mMIPTG (final concentration) and induce at 16°C for 20h.
Embodiment 3
[0060] Construction and induced expression of embodiment 3 genetically engineered bacteria
[0061] The plasmid pET30-HHDH constructed in Example 1 was used to transform the expression host EscherichiacoliBL21(DE3). Use primers F_HHDH / R_HHDH for colony PCR to verify transformed recombinants. The verified genetically engineered bacteria is EcoH. EcoH was inoculated into the fermentation medium and cultured with constant temperature shaking for 16 hours. The culture conditions were 40° C. and 220 rpm. When the cell concentration grows to OD 600 When =1.2, add 0.8mMIPTG (final concentration) and induce at 22°C for 20h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com