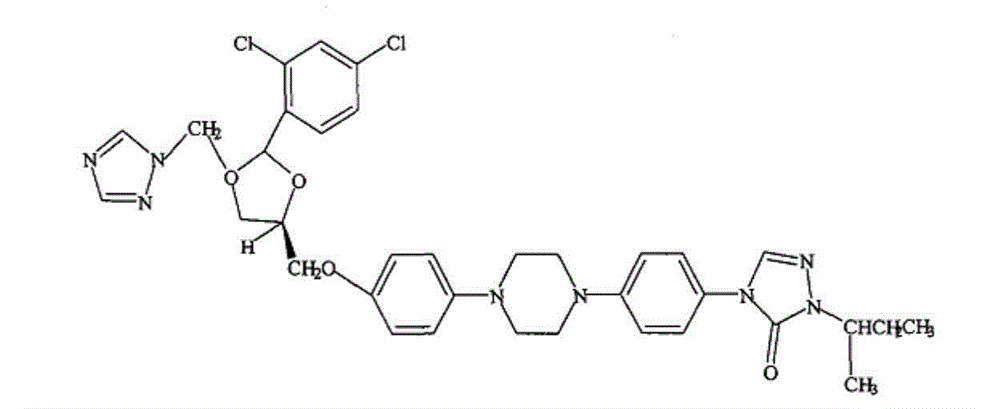
Itraconazole pharmaceutical composition, preparation method and application thereof
A technology of itraconazole and its composition, which is applied in the direction of pharmaceutical formulation, drug delivery, and medical preparations of non-active ingredients, etc., which can solve the problems of large individual differences in oral bioavailability, reduction of organic solvent residues, and large amount of organic solvents, etc. problems, to achieve the effect of easy swallowing, reduced amount of organic solvents, and improved solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 Formula composition and preparation method of the present invention
[0024] Formulation 1 (g / 1000 formulation units):
[0025]
[0026] Formulation 2 (g / 1000 formulation units):
[0027]
[0028] Formulation 3 (g / 1000 formulation units):
[0029]
[0030] Formulation 4 (g / 1000 formulation units):
[0031]
[0032] Preparation:
[0033] ①According to the formula ratio, weigh 100g of itraconazole, 130g of hydroxypropyl methylcellulose and 10g of release accelerator, and dissolve them in a mixed solution of 1000mL of dichloromethane and 1050mL of absolute ethanol to obtain a concentration of about 10% (W / W) of the drug solution, passed through a 80-mesh sieve, and placed for later use; ②Weigh 80g of the blank core, put it in the fluidized bed, spray the drug solution onto the surface of the blank core by using the fluidized bed bottom spray technology, and spray dry The suitable fluidized bed operating process parameters for the preparation o...
Embodiment 2
[0039] Embodiment 2 In vitro dissolution test
[0040] experiment method:
[0041] The samples of Example 1 formula 1 to formula 4 and comparative example 1 formula 5 are carried out in vitro dissolution test, the specific operation process is as follows: get this product, according to the dissolution method (Chinese Pharmacopoeia in 2010 two appendix XC second method ), using 1000ml of hydrochloric acid solution (9→1000) as the dissolution medium, the rotation speed was 75 rpm, the sampling time was 45 minutes, and the content determination method was UV-visible spectrophotometry (Chinese Pharmacopoeia 2010, Appendix IVA of Part II), and the determination The wavelength is 255nm.
[0042] The measurement results are shown in Table 1.
[0043] Table 1 In vitro dissolution test results
[0044]
[0045] The results of the in vitro dissolution test showed that the dissolution rate of itraconazole pellets added with a release enhancer was significantly increased in 45 minut...
Embodiment 3
[0046] Example 3 In vivo bioequivalence test
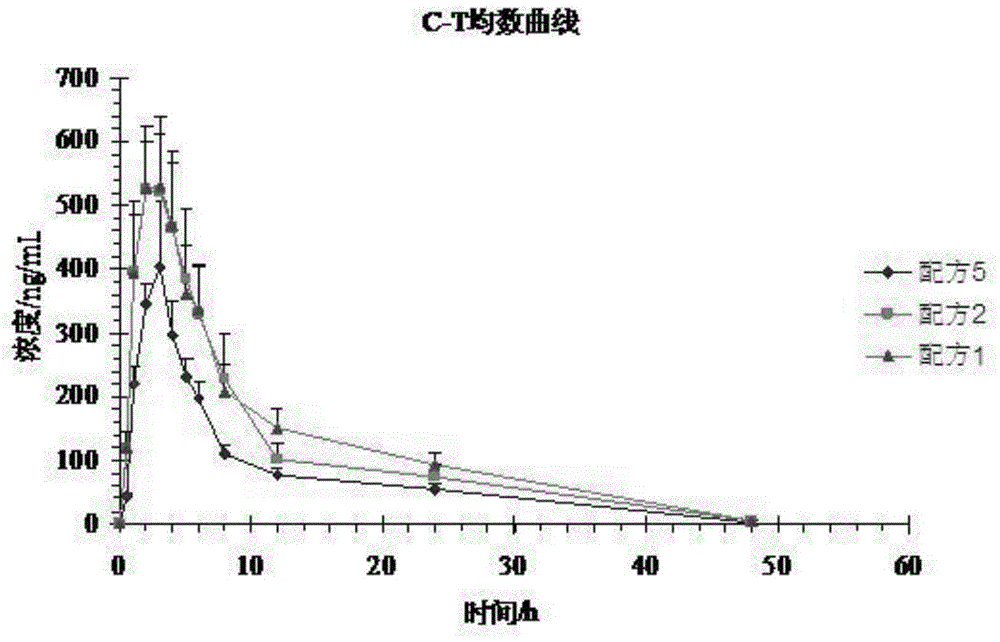
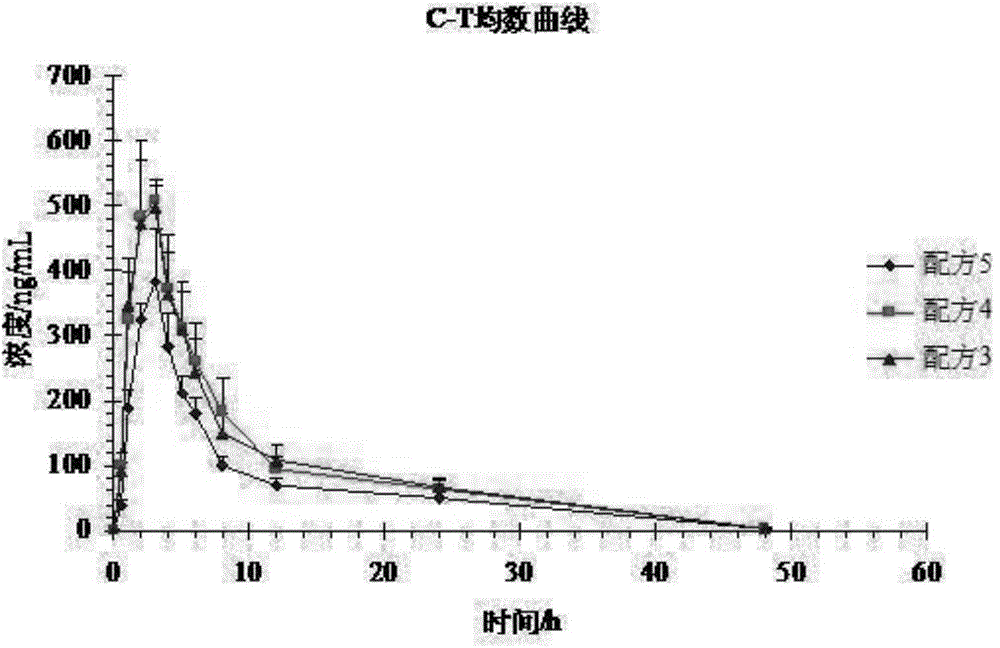
[0047] experiment method
[0048] The samples of formula 1 to formula 4 of embodiment 1 and formula 5 of comparative example 1 were subjected to bioavailability test in vivo, and the specific operation process was as follows:
[0049] Test object:
[0050] There were 24 Beagle dogs, half male and half male, and the weight of Beagle dogs was 10.68±0.51 kg.
[0051] Test plan:
[0052] Experiment 1: A self-controlled randomized cross-over trial (3 preparations, 3 cycles, double 3×3 Latin square test) design was adopted, and 12 Beagle dogs were cross-administered orally orally, and the dosage was based on the active prescription. Formula 1 (100mg) , formula 2 (100mg), formula 5 (100mg). 3 mL of blood was collected from the canine leg vein before and after administration and at 0.5, 1.0, 2.0, 3.0, 4.0, 5.0, 6.0, 8.0, 12.0, 24.0, and 48.0 hours, and placed in a heparinized centrifuge tube at 3500 r / min Centrifuge for 15 minutes to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com