Medicament for treatment of lung diseases and application thereof
A technology for pulmonary diseases and medicines, applied in the field of compositions containing guarene and/or aristolochne
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: the preparation of guurrene, aristolochne
[0016] 1) After crushing 5kg of Guangfangji or Xugufeng dried whole herb, add water 4 times the mass of the medicinal material, heat in a steam distillation device at 100°C, and distill for 2 hours. It can be separated from the fractionation tube of the distillation device to obtain about Medicinal quality 0.9% essential oils of volatile oils. The obtained essential oil was dried and filtered with anhydrous sodium sulfate.
[0017] 2) Pack the column with 100-140 mesh silica gel by wet method, add dehydrated volatile oil with 25% weight of silica gel, elute with petroleum ether below 50°C, collect the eluent in turn, and check with thin layer chromatography to find out the olefin part in the volatile oil After removing to a trace amount, use ethyl acetate-petroleum ether (25:100→100:0) gradient elution instead, detect the chromatographic fractions by silica gel thin layer, collect every 20mL fractions, and use p...
Embodiment 2
[0018] Embodiment 2: anti-acute pneumonia drug efficacy test
[0019] 1 Materials and methods
[0020] 1.1 Animals and grouping
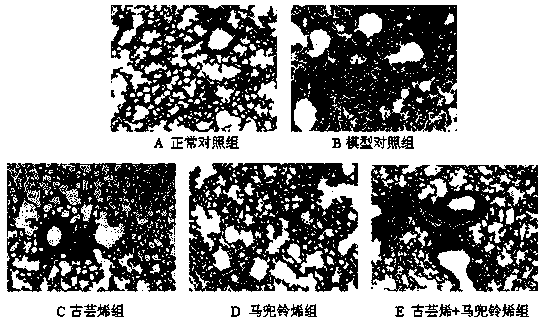
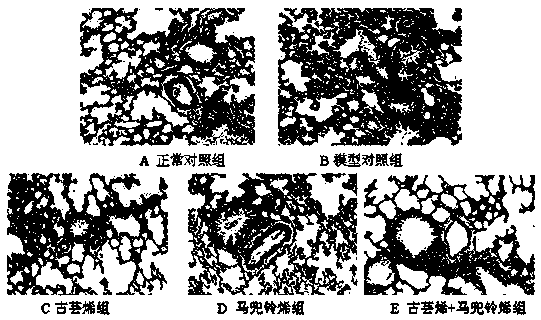
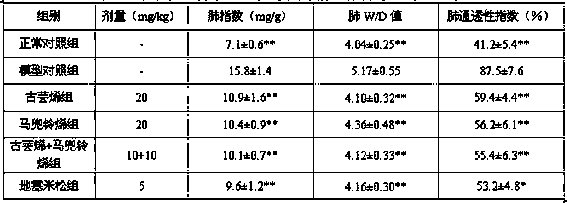
[0021] Clean-grade ICR mice, 18-22 g, were provided by the Experimental Animal Center of Zhejiang Province, animal production license number: SCXK (Zhejiang) 2014-0001. They were randomly divided into 6 groups according to their body weight: normal control group, model control group, dexamethasone group, guaranene group, aristolochne group and guaranene + aristolochne combined administration group, 10 rats in each group, Half and half male and half male, wherein guabrene and aristolochne were prepared by the method in Example 1.
[0022] 1.2 Model establishment and administration
[0023] Except for the normal control group which was given intravenous injection of the same dose of normal saline, the mice in the other groups were given tail vein injection of 5 mg / kg lipopolysaccharide (LPS). Oral administration was started 5 days before modeling....
Embodiment 3
[0042] Embodiment 3: anti-asthma efficacy test
[0043] 1 Experimental materials
[0044] Clean-grade Babl / c mice, half male and half female, 18-22 g, provided by Shanghai BK Company, animal license number: SCXK (Shanghai) 2013-0016.
[0045] Ovalbumin (batch number JS10710), Shanghai Jinsui Biotechnology Co., Ltd.; prednisone acetate (batch number: 120807), Zhejiang Xianju Pharmaceutical Co., Ltd.
[0046] 2 Experimental methods
[0047] 2.1 Model establishment and grouping
[0048] Babl / c mice were adaptively fed for 1 week and fed standard pelleted diet. Then they were divided into random groups, with 8 rats in each group, half male and half male. They were respectively set as normal control group, asthma model group, prednisone administration group, guacrene group, aristolochne group and guarrene + aristolochne combined administration group. Modeling method: Each mouse in the model group was sensitized by intraperitoneal injection of 0.2 mL PBS solution containing 20 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com