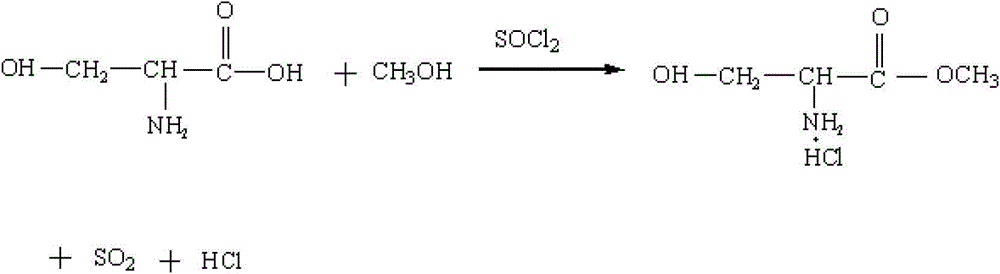
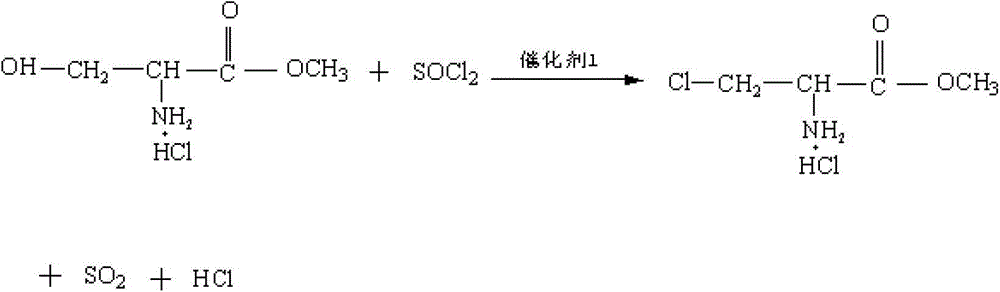
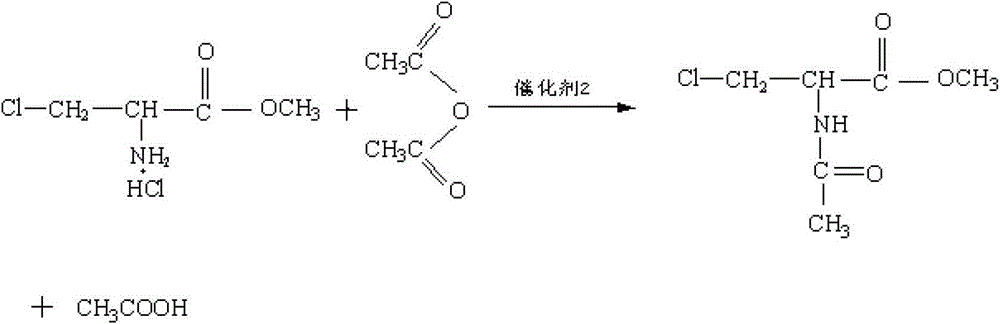
Method for preparing N-acetyl-beta-chlorine-L-alanine methyl ester
A technology of alanine methyl ester and acetyl, which is applied in the field of preparation of ramipril intermediates, can solve the problems of low reaction yield, many by-products, environmental pollution by tail gas, etc., and achieves low cost, few by-products, and high reaction yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Pump 300 kg of methanol into the reaction pot, start adding 90 kg of thionyl chloride dropwise, and control the temperature of the material in the pot not to exceed 20°C. After the dropwise addition, 65 kg of serine was added to the reaction pot, heated and refluxed for 10 hours, and the tail gas generated by the reaction was absorbed with 30% sodium hydroxide solution. After the reflux reaction was completed, the reaction solution was concentrated under reduced pressure to about 200 kg, lowered to normal temperature, and centrifugally filtered. Dry the precipitate at 60°C. The filtrate is concentrated, and the concentrated semi-solid is used as the masterbatch for the next batch of reaction in this step, and the distilled liquid is neutralized and separated by sodium hydroxide and reused as methanol.
[0038] (2) Pump 500 kg of dichloroethane into the reaction pot, add the above-mentioned precipitation drying material, and then pump in 60 kg of thionyl chloride an...
Embodiment 2
[0041] (1) Pump 1,500 kg of methanol into the reaction pot, start adding 450 kg of thionyl chloride dropwise, and control the temperature of the material in the pot to not exceed 20°C. After the dropwise addition, 325 kg of serine was added to the reaction pot, heated and refluxed for 15 hours, and the tail gas produced by the reaction was absorbed with 30% potassium hydroxide solution. After the reflux reaction was completed, the reaction solution was concentrated under reduced pressure to about 1000 kg, cooled to room temperature, and centrifugally filtered. Dry the precipitate at 60°C. The filtrate is concentrated, and the concentrated semi-solid is used as the masterbatch for the next batch of reaction in this step, and the distilled liquid is neutralized and separated by sodium hydroxide and reused as methanol.
[0042] (2) Pump 2,500 kg of dichloroethane into the reaction pot, add the precipitating drying material, and then pump in 300 kg of thionyl chloride and 0.03 kg...
Embodiment 3
[0045] (1) Pump 3,000 kg of methanol into the reaction pot, start to add 900 kg of thionyl chloride dropwise, and control the temperature of the material in the pot to not exceed 20°C. After the dropwise addition, 650 kg of serine was added to the reaction pot, heated and refluxed for 10 hours, and the tail gas generated by the reaction was absorbed with 30% sodium hydroxide solution. After the reflux reaction was completed, the reaction solution was concentrated under reduced pressure to about 2000 kg, cooled to room temperature, and centrifugally filtered. Dry the precipitate at 60°C. The filtrate is concentrated, and the concentrated semi-solid is used as the masterbatch for the next batch of reaction in this step, and the distilled liquid is neutralized and separated by sodium hydroxide and reused as methanol.
[0046] (2) Pump 5,000 kg of dichloroethane into the reaction pot, add the precipitating dry material, and then pump in 600 kg of thionyl chloride and 60 kg of dim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com