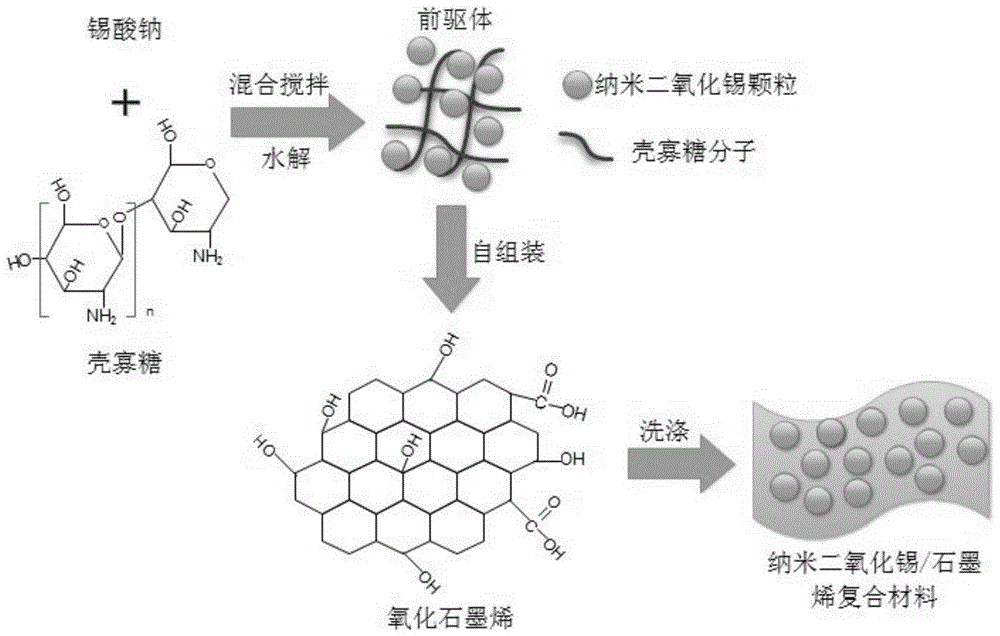
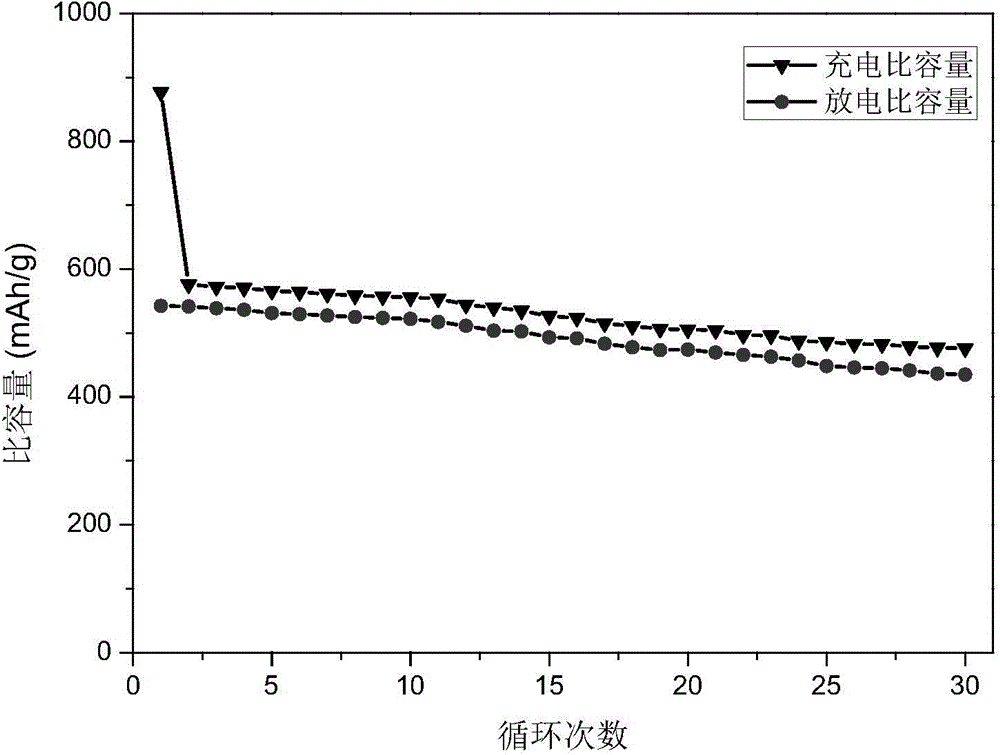
Method of preparing stannic oxide/graphene composite lithium ion battery anode material under the assistance of chitosan oligosaccharide self-assembly
A graphene composite and lithium-ion battery technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems of harsh reaction conditions, polluted environment, energy consumption, unfavorable environmental protection, etc. Low power consumption, environment-friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Dissolve 3 g of sodium stannate in 80 ml of deionized water, add 5 g of chitosan oligosaccharide (degree of polymerization 6, degree of deacetylation 60%), mix and stir to obtain a self-assembled precursor of nano-tin dioxide modified by chitosan oligosaccharide. 0.045 g of graphene oxide powder was added to the precursor to obtain a mixed solution, and then stirred at 20° C. for 20 hours for a mixed reaction. The resulting product is put into a centrifuge for separation, and the precipitate is repeatedly washed with deionized water. After washing, it is dried in a vacuum oven at 50°C for 8 hours, and the obtained solid powder is the oligochitosan self-assembly aid of the present invention. Preparation of tin dioxide / graphene composite lithium ion battery negative electrode material.
Embodiment 2
[0024] Dissolve 1.8g sodium stannate in 80ml deionized water, add 2.8g chitosan oligosaccharide (polymerization degree 20, deacetylation degree 95%), mix and stir to obtain the nano-tin dioxide self-assembly precursor modified by chitosan oligosaccharide. 0.085 g of graphene oxide powder was added to the precursor to obtain a mixed solution, and then stirred at 40° C. for 10 hours for a mixed reaction. The resulting product is separated by filtration, and the precipitate is repeatedly washed with deionized water. After washing, it is dried in a vacuum oven at 30°C for 10 hours. The obtained solid powder is the chitosan oligosaccharide self-assembly-assisted preparation Tin / graphene composite lithium-ion battery anode material.
Embodiment 3
[0026] Dissolve 3.5g sodium stannate in 60ml deionized water, add 5.5g chitosan oligosaccharide (polymerization degree 6, deacetylation degree 50%), mix and stir to obtain the nano-tin dioxide self-assembly precursor modified by chitosan oligosaccharide. 0.14 g of graphene oxide powder was added to the precursor to obtain a mixed solution, and then stirred at 60° C. for 6 hours for a mixed reaction. Put the obtained product into a centrifuge for separation, and repeatedly wash the precipitate with deionized water, dry it in a vacuum oven at 80°C for 5 hours after washing, and the obtained solid powder is the oligochitosan self-assembly aid of the present invention. Preparation of tin dioxide / graphene composite lithium ion battery negative electrode material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com