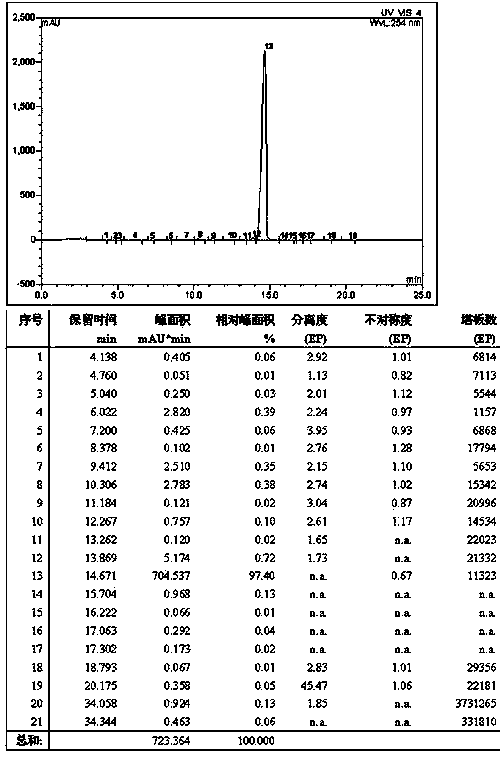
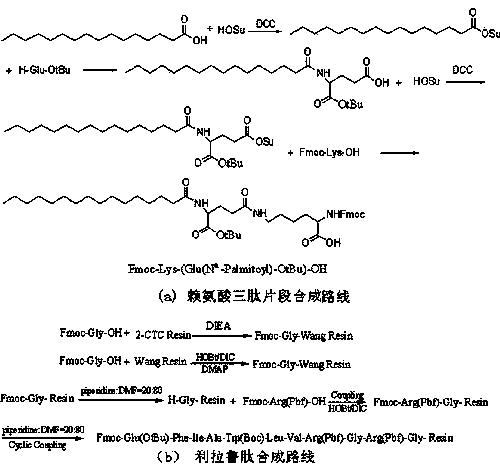
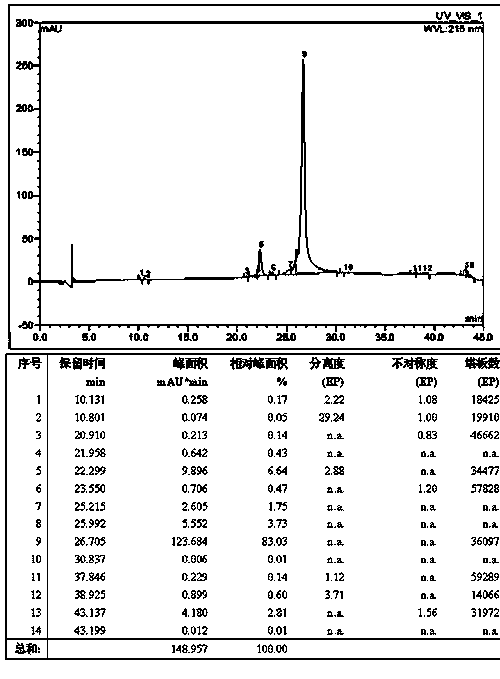
Preparation method of liraglutide
A technology of liraglutide and synthetic method, which is applied in the field of preparation of polypeptide drugs, can solve the problems of many impurities, low yield, and unsuitability for industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Example 1: Palmitoyl-OSU activation fat synthesis
[0092] Called 256.42g positive hexisc acid (1.0 mol), 138.10g HOSU (1.2mol) Add 2000ml THF, add 247.56g DCC (1.2mol) under the ice water bath, react for 1 hour, react to room temperature for 3 hours, and response for 3 hours.Filter, dry the mother liquid, dissolve with DCM, filtrate, washed sodium saturated sodium bicarbonate 3 times, 2 times of pure water, 2 times of anti-extract, combined with organic phase, dry sodium carbonate, dried, 0-5 ° C ice ethanol, 0-5 ° C ice ethanol3 times heavy crystal, filtering, solid oil pump to get 314.62G Palmitoyl-Osu activated lipids, with 89%income.
Embodiment 2
[0093] Example 2: Palmitoyl-Glu-OTBU synthesis
[0094] It is said that 101.62g H-Glu-OTBU (0.5mol) and 79.50G NA 2 CO 3 (0.75mol) Add to a mixed solution of 500ml of water and 500ml THF to dissolve, which is called 176.75G Palmitoyl-OSU (0.5mol) and adds to 500ml THF.Dilute hydrochloric acid regulate pH to 7, rotate the THF, and then adjust the pH to 3.Get a lot of white precipitation and filter.The obtained white precipitation is required with 0-5 ℃ ice ethanol.The solid oil pump was pulled by 192.11g Palmitoy-Glu-OTBU, with a revenue of 87%.
Embodiment 3
[0095] Example 3: Palmitoyl-Glu (OSU) -s' synthesis of OTBU
[0096] Called 88.33G Palmitoyl-Glu-OTBU (0.2mol), 27.62g HOSU (0.24mol) Add 1000ml THF, add 49.51g DCC (0.24mol) under the ice water bath, respond to 1 hour, heating to room temperature for 3 hours, reaction to the reaction.Filter, dry the mother liquid, dissolve with DCM, filtrate, washed sodium saturated sodium bicarbonate 3 times, 2 times of pure water, 2 times of anti-extract, combined with organic phase, dry sodium carbonate, dried, 0-5 ° C ice ethanol, 0-5 ° C ice ethanol3 times heavy crystal, filtering, solid oil pump to get 94.81g Palmitoyl-Glu (OSU) -ebu activated lipid with 88%income.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com