Semen ziziphi spinosae alkaloid monomer composition zizyphusine, and preparation method and application thereof
A technology of jujube seed and alkaloids, applied in the directions of pharmaceutical formulation, drug combination, drug delivery, etc., can solve the problems of large dosage of traditional Chinese medicine and many side effects of antidepressants, achieve no toxic side effects, prevent and treat insomnia, Preparation method for stable and reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for extracting and separating plum alkali from Suanzaoren, the method comprising the following steps:
[0035] Impregnate 120g of jujube seed with 7 times and 4 times (w / v) 0.5% hydrochloric acid for 2 times, each time for 24 hours, combine the filtrate, centrifuge to get the supernatant, and absorb it with X-5 macroporous adsorption resin column, distilled water After elution, elute with 70% ethanol solution, and recover ethanol by distillation under reduced pressure to obtain the total alkaloids of Suanzaoren (3.07g).
[0036] The alkaloids of Jujube Seed are dissolved in distilled water (w / v=5:1), and the pH is adjusted to 12 with ammonia water. Extract with n-butanol solution, distill the organic layer under reduced pressure, and recover the solvent to obtain the n-butanol layer extract. The n-butanol layer extract was further separated by neutral alumina column chromatography, and the elution gradient was: 100% methanol, methanol-water (1:1), and 100% wat...
Embodiment 2
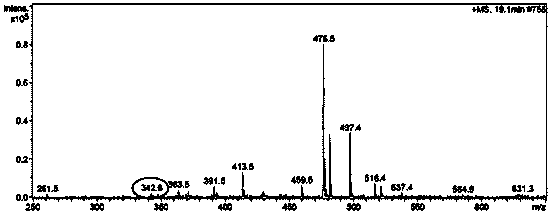
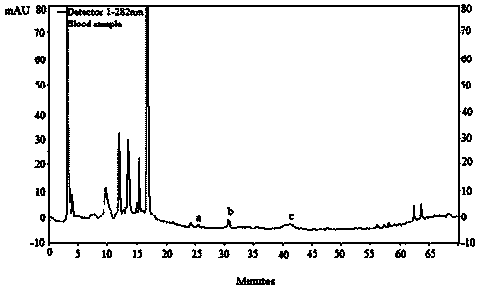
[0039] Serum Medicinal Chemistry Method Plumbine is the active ingredient of jujube seed alkaloids entering the blood.
[0040] 1. Experimental animals
[0041] Healthy male ICR mice (SPF grade, body weight 20±2g); purchased from the Experimental Animal Center of the Institute of Radiological Medicine, Chinese Academy of Medical Sciences, certificate number: SCXK (Beijing) 2009-0004; raised in the laboratory for 3 days before the experiment, and adapted to environment. The room temperature is 22±2°C, and the relative humidity is 65% to 70%. Sunlight for 12 hours, free diet and water intake.
[0042] 2. Methods and results
[0043] 2.1 Preparation and administration of medicinal liquid
[0044]Precisely weigh the extract of the alkaloid part of Suanzaoren, dissolve it in distilled water, and make a 20mg / mL liquid medicine. 10 mice were randomly numbered, among which Nos. 1 to 5 were the model group, and Nos. 6 to 10 were the administration group; the model group was given ...
Embodiment 3
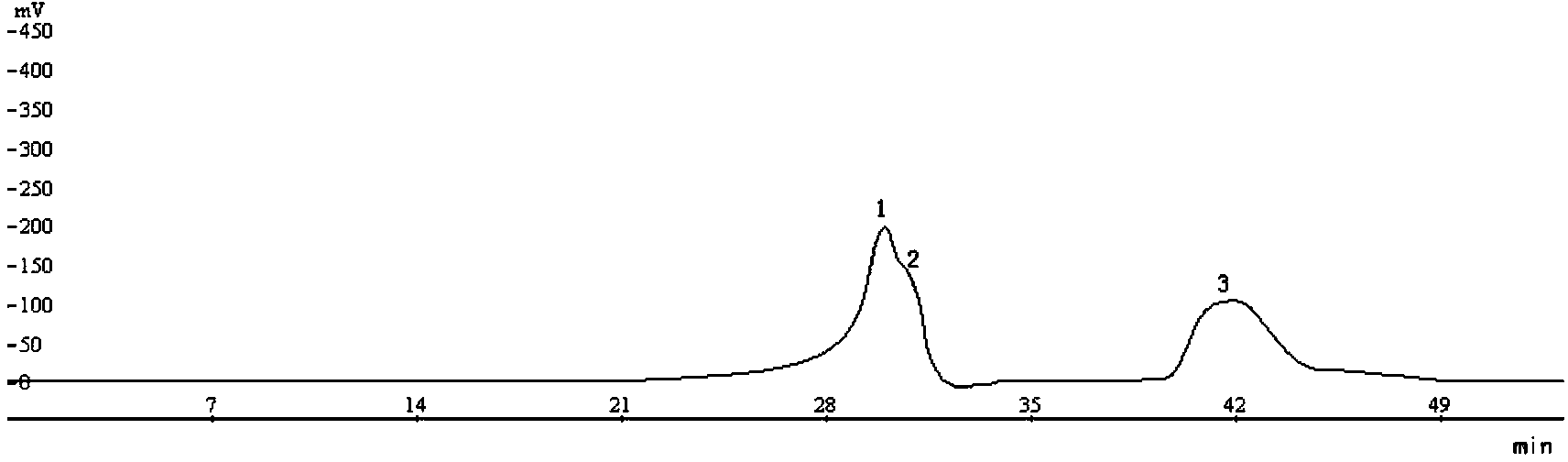
[0050] Pharmacodynamic test of alkaloid monomer Libine in jujube seed
[0051] 1. Materials
[0052] 1.1 Experimental animals: Experimental animals: 40 male ICR mice (SPF grade, body weight 20±2g) were purchased from Beijing Huafukang Biotechnology Co., Ltd., Chinese Academy of Medical Sciences, Chinese Union Medical University Institute of Experimental Animals, certificate number: SCXK (Beijing) 2009-0008. Raised in the laboratory for 3 days before the experiment to adapt to the environment. The room temperature is 22±2°C, and the relative humidity is 65% to 70%. Light for 12 hours, free to eat and drink.
[0053] 1.2 Drugs and reagents: Suanzaoren (Anguo Qirui Traditional Chinese Medicine Co., Ltd., batch number: 100607, identified by the Department of Medicine, Tianjin Medical University); Jieyu Anshen Granules (Jilin Minmintang Pharmaceutical Co., Ltd., batch number: 20100103075), hydrochloric acid Venlafaxine extended-release capsules (Effexor, Wyeth, Ireland, batch n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com