Polypeptide for inhibiting hiv, pharmaceutical composition and use thereof
A composition and drug technology, applied in the field of its pharmaceutical composition and HIV-inhibiting polypeptide, can solve the problems of short sequence and high synthesis cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: Preparation of polypeptide 1
[0128] Standard Fmoc solid-phase peptide synthesis method is used. All polypeptide sequences are amidated at the C-terminus and acetylated at the N-terminus in accordance with the conventions of polypeptide synthesis (persons in the art know that these modifications have no fundamental impact on polypeptide activity). Choose RinkAmide resin, and peptides extend from C-terminal to N-terminal. Condensing agent is HBTU / HOBt / DIEA. The deprotection agent is piperidine / DMF solution. The lysing agent is TFA, and the crude peptide is dissolved in water and lyophilized for storage. Separation and purification by medium pressure liquid chromatography or HPLC, pure peptide content> 95%. Matrix-assisted laser analysis time-of-flight mass spectrometry (MALDI-TOF-MS) determines the molecular weight of peptides.
[0129] The conditions for microwave peptide synthesis are as follows:
[0130] Amino acid: 0.2M DMF solution; activator: 0.45M HBTU...
Embodiment 2-12
[0137] Example 2-12: Preparation of polypeptide 2-12
[0138] The preparation was carried out with reference to the method in Example 1. The specific amino acid sequences are shown in Table 1 respectively. Its molecular weight and purity are shown in Table 2 above.
Embodiment 13
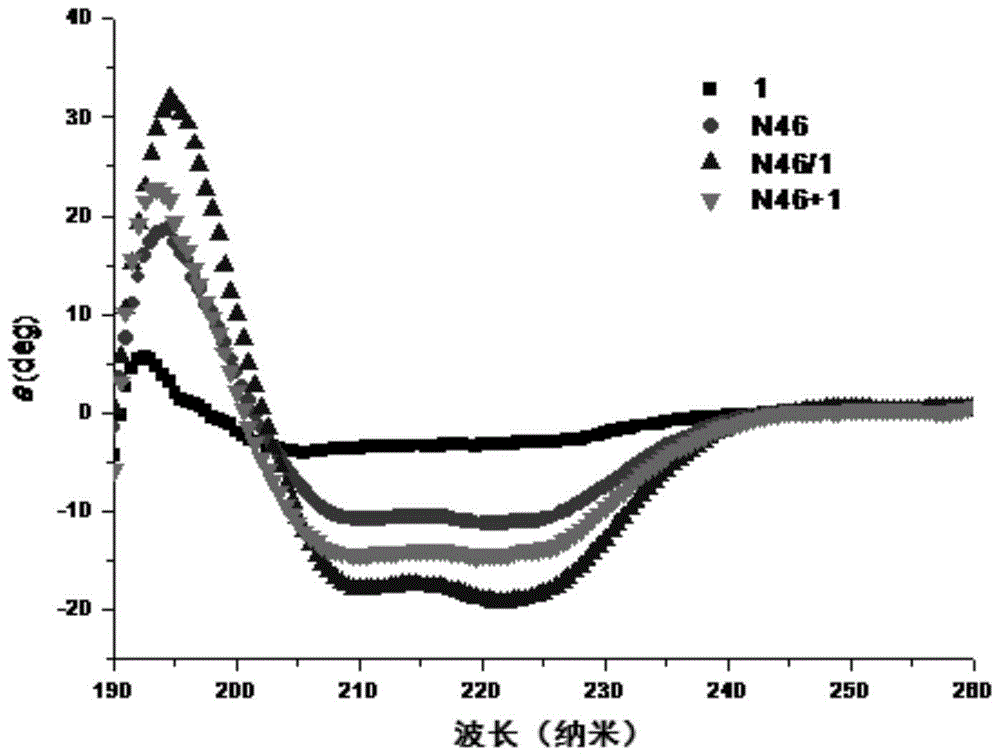
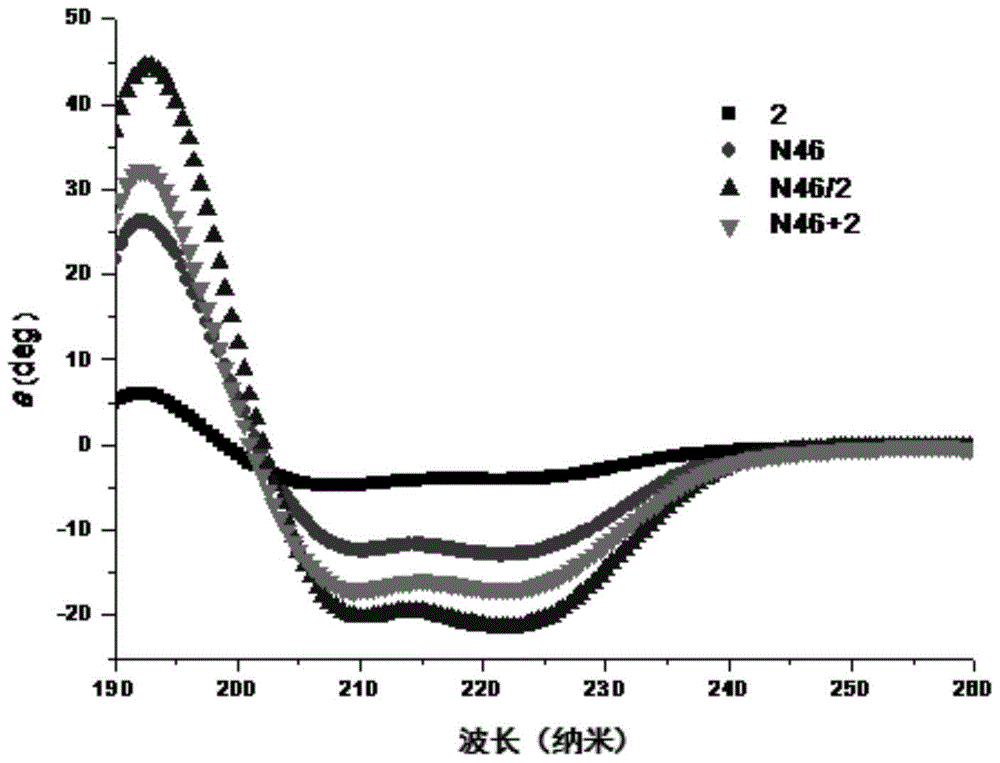
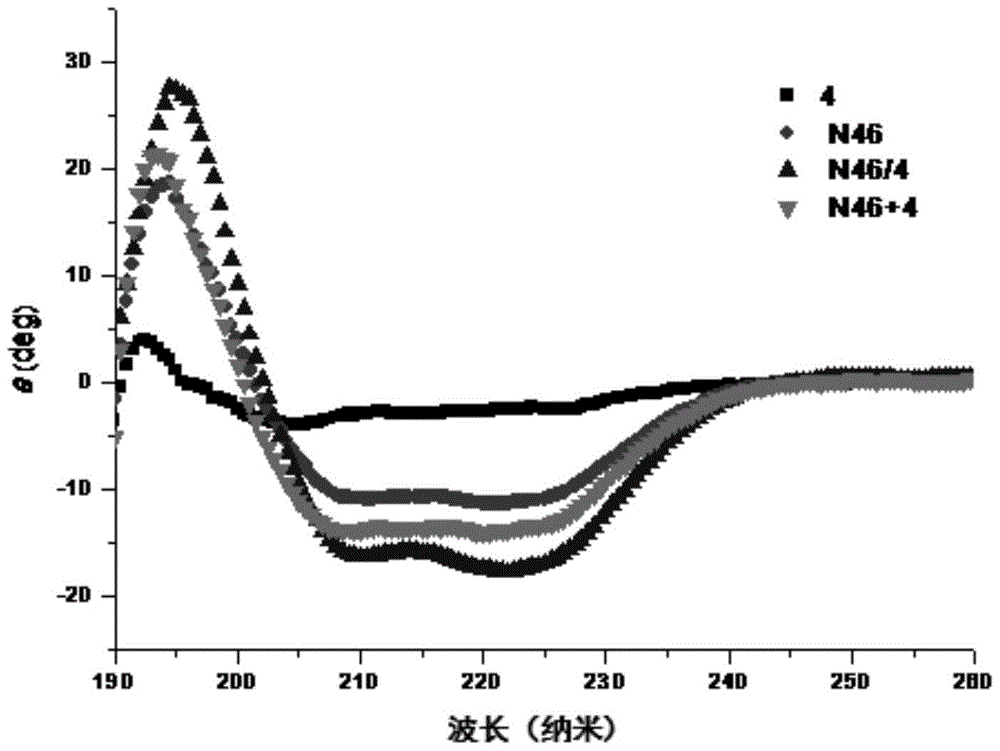
[0140] Example 13: Detection of anti-HIV-1 cell-cell fusion activity of polypeptide
[0141] The inventors used HIV-1 Env-mediated cell-cell fusion model to determine the activity of the designed polypeptide. The target cells are TZM-bl cells (provided by NIH AIDS Reagents and Reference Projects in the United States, catalog number 8129), which express CD4 T-cell receptors and chemokine co-receptors CCR5 and CXCR4 on their surface, which can be recognized by HIV-1Env. At the same time, the luciferase reporter gene is also transcribed in the cell, but does not contain the promoter of this gene, so the background expression of luciferase in a single cell is very low. The effector cells are HL2 / 3 cells (provided by NIH AIDS Reagents and Reference Project, catalog number 1294), which express HIV-1 Env on the surface, and Env attacks the target cells to complete cell fusion. At the same time, the luciferase report is also transcribed in the cell The promoter of the gene. The two ki...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com