Method for preparing highly pure L-tertiary leucine through biological process
A tertiary leucine biosynthesis technology, applied in the direction of microorganism-based methods, biochemical equipment and methods, microorganisms, etc., can solve the problems of high price and high cost, and achieve the effect of low cost, low pollution and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
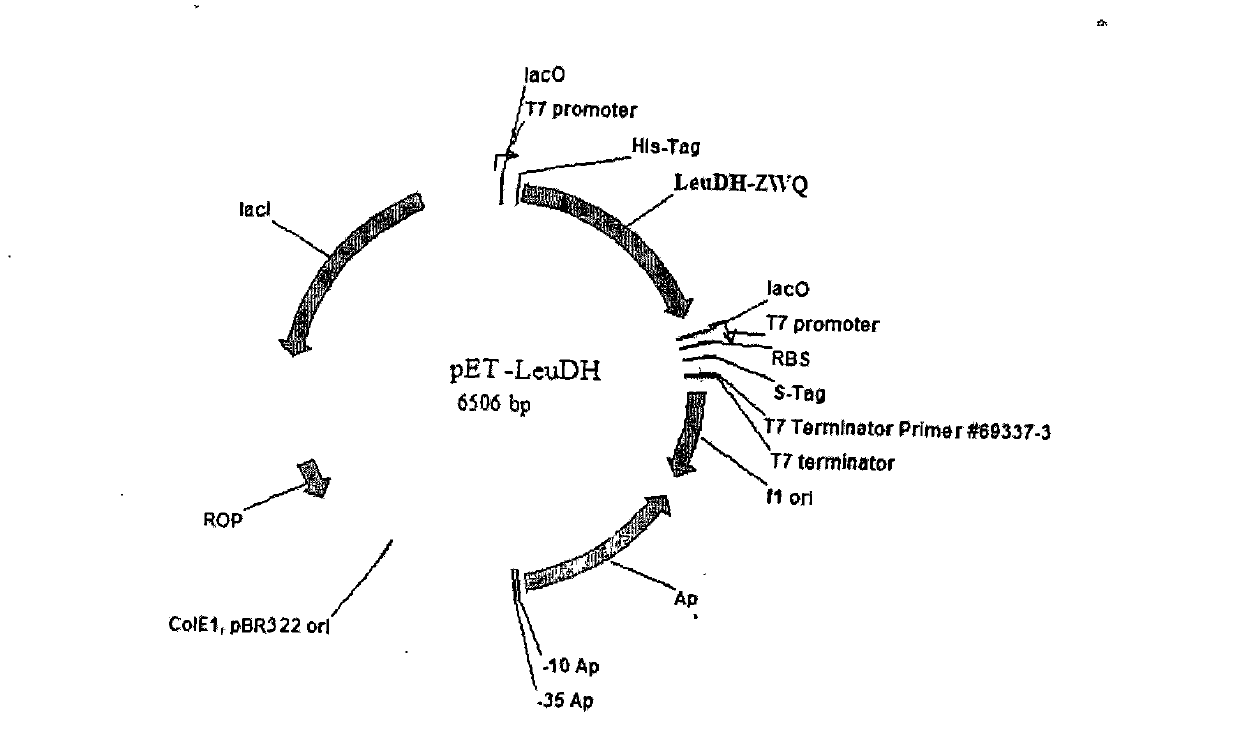
[0026] Embodiment 1, the construction of two kinds of recombinant bacteria containing leucine dehydrogenase gene and formate dehydrogenase gene
[0027] Genomic DNA was extracted from Bacillus cereus and Candida boidinii respectively, and the target gene fragments of fdh (formate dehydrogenase) and LeuDH (leucine dehydrogenase) were obtained by PCR method respectively. Gene fragments; then, express the fdh target gene and LeuDH target gene through the plasmid pETDuet-1. For detailed operation methods, see Organic Process Research & Development, 2006, 10, 666-669.
Embodiment 2
[0028] Embodiment 2, self-induced fermentation culture of genetically engineered bacteria (two kinds of recombinant bacteria culture methods are the same). Add 100 μl of genetically engineered bacteria seed solution into 10 ml LB medium containing 100 μg / ml kanamycin, and cultivate overnight at 37° C. and 200 rpm. Take 400 μl of the bacterial solution in the logarithmic growth phase and insert it into 40 ml of the following medium containing 100 μg / ml kanamycin, and incubate at 37° C., 200 rpm for 12 hours. The medium composition is: 1% tryptone, 0.3% yeast extract, 25mM Na 2 HPO 4 , 25mM KH 2 PO 4 , 50mM NH 4 Cl, 5mM Na 2 SO 4 , 2mM MgSO 4 , 1.0% glycerol, 0.05% glucose, 0.25% lactose, 100 μM FeCl 3 .
Embodiment 3
[0029] Example 3, preparation of the recombinant cell suspension: the fermented broth was centrifuged (10000g, 5 minutes, 4° C.) to obtain crude bacteria, washed and suspended with pH6.0-7.0 phosphate buffer, used for the following experiments or stored in- 20°C for use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com