Construction method and application of potato x virus overexpression and bimolecular fluorescent complementation vector
A potato, overexpression technology, applied in the field of genetic engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Extraction of plant total RNA
[0037] Take about 0.1g of the upper leaves of PVX-infected tobacco plants, grind them thoroughly in liquid nitrogen, add 1mL TRIZOL, vortex, incubate at room temperature for 5min, add chloroform, vigorously vortex for 15s, incubate at room temperature for 3min, and centrifuge at 13000r / min for 15min. Carefully draw the supernatant, add 1 / 2 volume of isopropanol, let stand for 10 minutes, and centrifuge at 13000r / min for 10 minutes. The precipitate was washed with 75% ethanol and centrifuged at 13000r / min for 5min. The precipitate was redissolved with 50 μL DEPC water and stored at -80°C for later use.
Embodiment 2
[0038] Example 2: Construction of Potato Virus X Infectious Clones
[0039] Using the viral RNA obtained in Example 1 as a template, reverse transcription was performed with random primers. According to the existing restriction enzyme map of the entire genome of potato virus X, it can be amplified in three parts, and assembled into a PVX full-length cDNA clone after enzyme digestion. First, the 35S promoter was fused to the fragment PVX5' untranslated region (UTR) to the upstream of the nt543-BamHI restriction site by Overlap-PCR, and the inventor named the fragment p35S-543; PCR amplified 543-BamHI The fragment between the restriction site and the 3349-EcoRI restriction site, the inventor named the fragment as segment 543-3349; PCR amplification of the fragment from the 3349-EcoRI restriction site to the ploy (A) tail , the inventors named the fragment as fragment 3349-polyA.
[0040] Using the total plant RNA as a template, Moloneymurineleukavirus reverse transcriptase (Pr...
Embodiment 3
[0048] Embodiment 3: the preparation of potato X virus overexpression vector
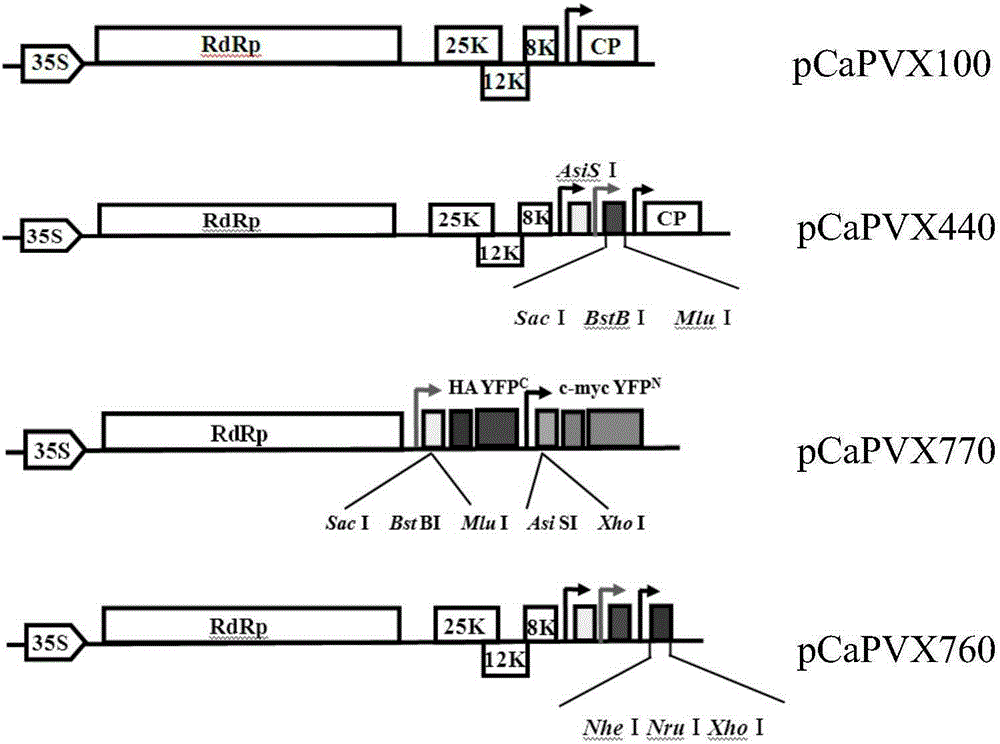
[0049] Primer 11 and primer 12 amplify the CP promoter 209bp of TMV plasmid, primer 7 (with ApaI restriction site) and primer 14 amplify a 599bp band, primer 13 and primer 8 (with SmaI restriction site) Amplify the 864bp band, recover the 3 bands obtained above, mix them, and use primer 7 and primer 8 to amplify, use Overlap-PCR to insert the TMVCP promoter between the PVXCP promoters, and start at TMVCP Insert a multiple cloning site (AsisI and SacI, BstBI, MluI in turn) before and after the son. ApaI upstream of the TGB gene and SmaI downstream of the 3'-UTR were double-digested and inserted between the corresponding restriction sites of pCaPVX100 to construct a vector capable of simultaneously expressing two foreign genes. The expression vector was named pCaPVX440 ( figure 1 ), the sequence is shown in SeqIDNo:32.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com