Method for screening H7N9 biomarkers in in-vitro blood plasma, and its application
A biomarker, plasma technology, applied in biological testing, biological material analysis, material testing products, etc., can solve unpublished reports, high mortality of severe H7N9 infection, and there is no biological predictor of disease severity and prognosis. Marker reporting and other issues to achieve excellent sensitivity and specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0053] Specific schemes of the present invention are provided by the following examples:
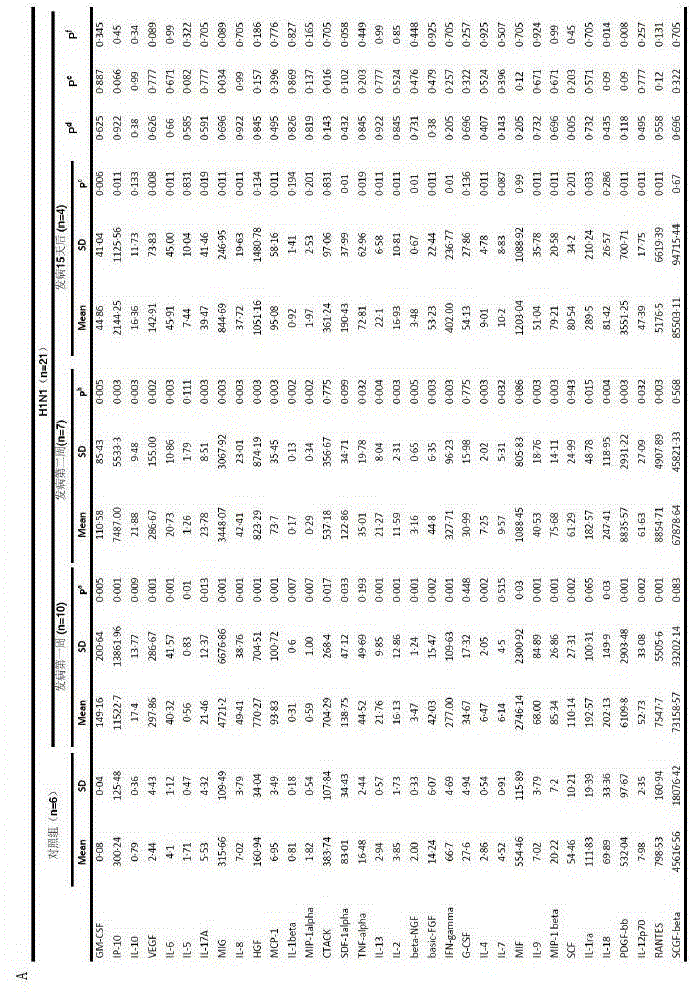
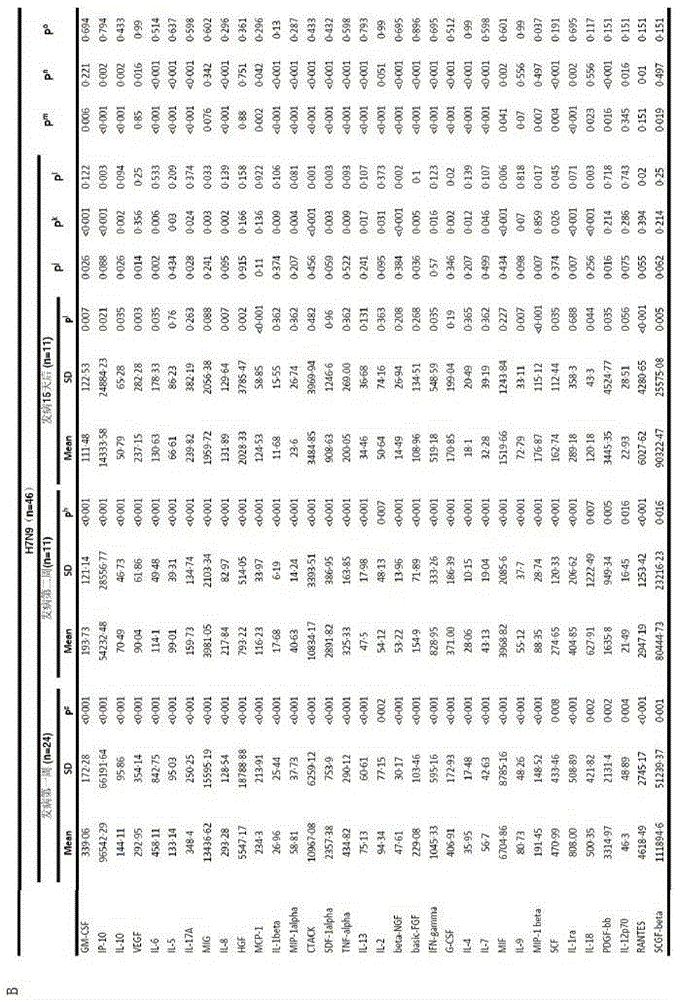
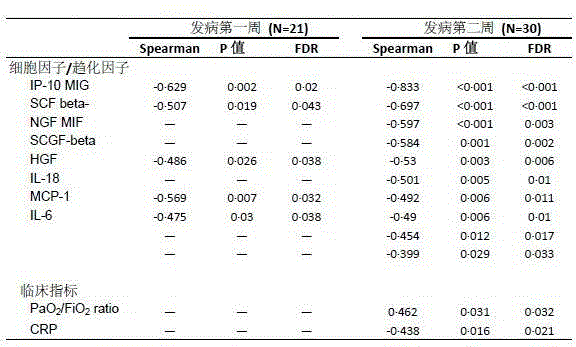
[0054] Determination of plasma immune factors in H7N9 patients
[0055] (1) Experimental samples
[0056] 1. 46 H7N9 patients, 35 patients within 2 weeks of onset, and 80 plasma samples. The average age was 61 years, of which 22 were male.
[0057] 2. There were 21 H1N1 patients with an average age of 53.9 years, including 14 males.
[0058] 3. Six control samples were taken from healthy volunteers.
[0059] (2) Experimental materials
[0060] 1. Reagent Bio-Plex Pro Human Cytokine Array 27-Plex Group I, 21-Plex Group II, filter paper, 1ml pipette, 200ul pipette, 1ml sterile pipette tip, 200ul sterile pipette tip, 10ul sterile pipette tip 2, Instrument Luminex200, horizontal shaker, magnetic stand, 1.5ml EP tube, 2ml EP tube.
[0061] (3) Experimental steps
[0062] 1. Collection of cases
[0063] The diagnosis of H7N9 patients is based on the epidemiological contact history, clin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com