Low Residue Inorganic-Organic Hybrid Monolithic Matrix Immobilized Enzyme Reactor and Its Preparation
A technology of immobilized enzymes and organic hybridization, which is applied in the direction of immobilization on/in the organic carrier to achieve good preparation reproducibility, mild preparation conditions, and high-throughput protein enzymatic hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Preparation of hybrid silica gel monolithic matrix material: Weigh 540mg of PEG-10000, dissolve it fully with 5mL of 0.01M acetic acid, then add 1.8mL of tetramethoxysilane and 0.6mL of vinyltrimethoxysilane, and stir for 1h at 0°C , to make reaction solution A. Weigh 2 mg of ammonium persulfate, dissolve it in 200 μL of reaction solution A, then add 200 μL of absolute ethanol and 10 μL of methacrylic acid in sequence, and shake and sonicate for 5 minutes. After cooling, add 50 μL of water and 50 μL of 0.5M ammonia water, pour into a capillary after shaking, and put it in a 40°C water bath for 24 hours.
[0026] 2. Substrate surface modification: After taking out the monolithic column, wash the pore-forming agents such as PEG-10000 and other unreacted small molecules with water. Weigh 10 mg and 20 mg of EDC and NHS, dissolve them in 1 mL of 50 mM pH 5.0 phosphate buffered saline, and pass them through the monolithic column for 4 hours, then rinse the unreacted EDC a...
Embodiment 2
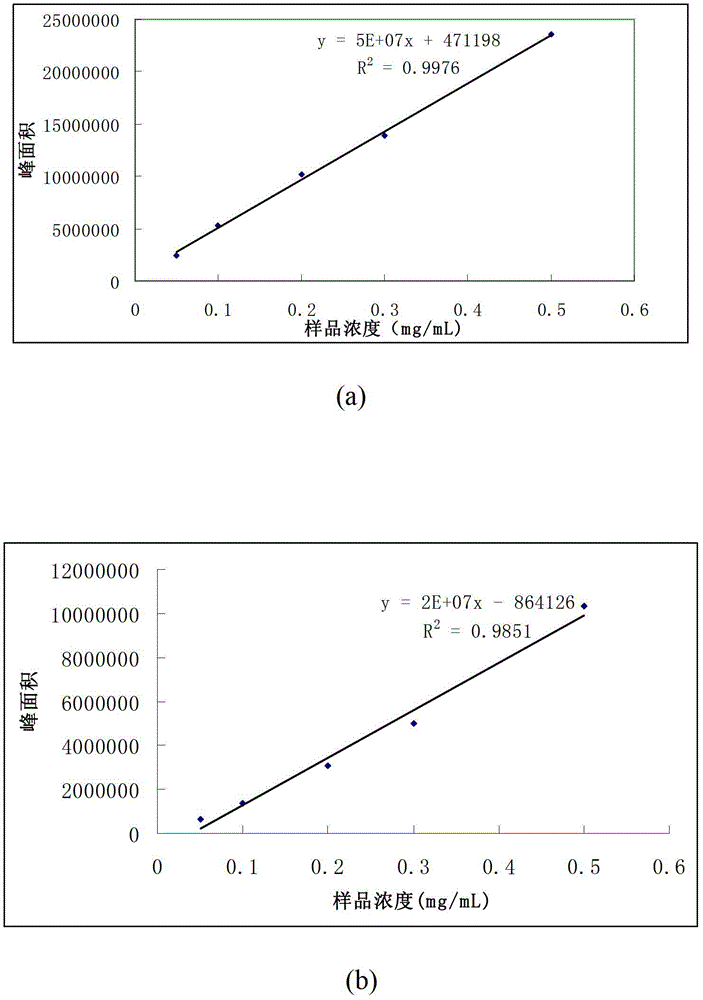
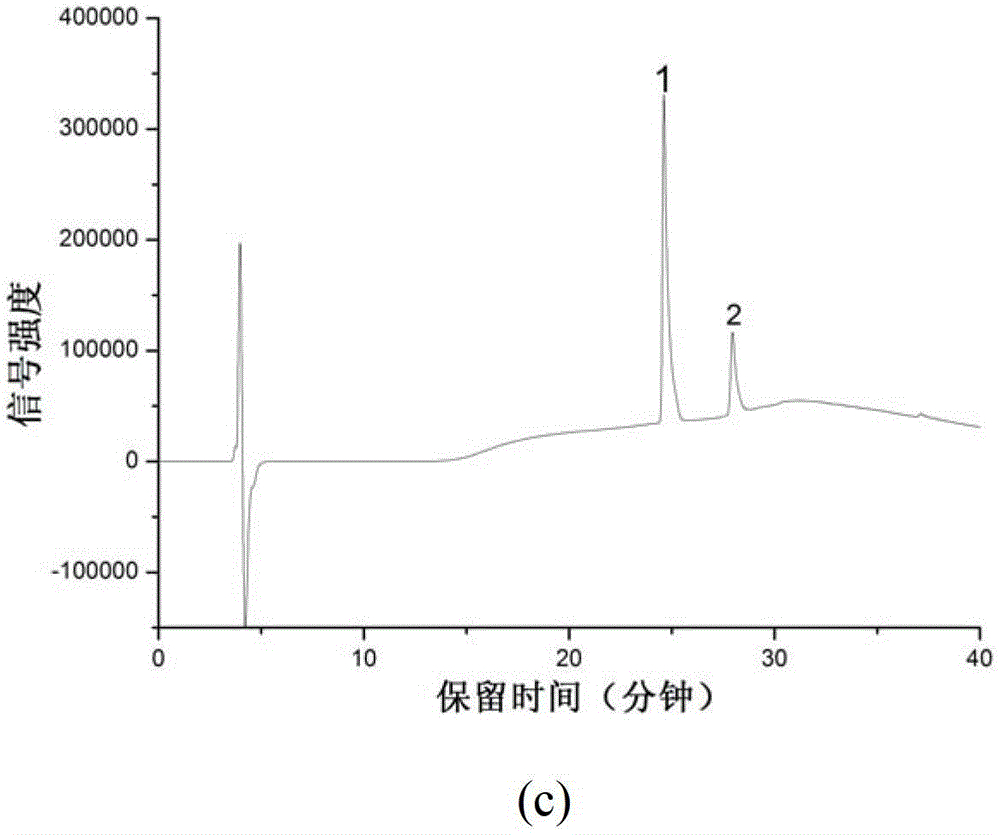
[0029] Investigation of the recovery rate of proteins and peptides on the hybrid silica monolithic material matrix modified with PEI: prepare 1 mg / mL each of peptide (DRVYHPHL) and BSA, dilute with 50 mM ammonium bicarbonate (pH8.0) respectively, and mix at a ratio of 1:1 , the concentration of the mixed solution is 50 μg / mL, 100 μg / mL, 200 μg / mL, 300 μg / mL, 500 μg / mL, and draw the standard curve of BSA (2a) and polypeptide (2b), the separation conditions are as follows: chromatographic column: Proteonavi column (4.6mm i.d×150mm, 5μ, ), mobile phase: A: 0.1%TFA+H 2 O;B: 0.1%TFA+ACN; Gradient: 0-5min, 5%B, 5-35min, 5%B-80%B; flow rate: 0.5mL / min, detection: 214nm; injection volume: 20μL. In order to investigate the adsorption of the matrix to the sample, a section of 5 cm long monolithic column was intercepted, and 100 μg / mL mixed sample was passed through at 1 μL / min, collected for 100 min, and then 20 μL was injected, and the chromatographic picture Such as figure 1 As s...
Embodiment 3
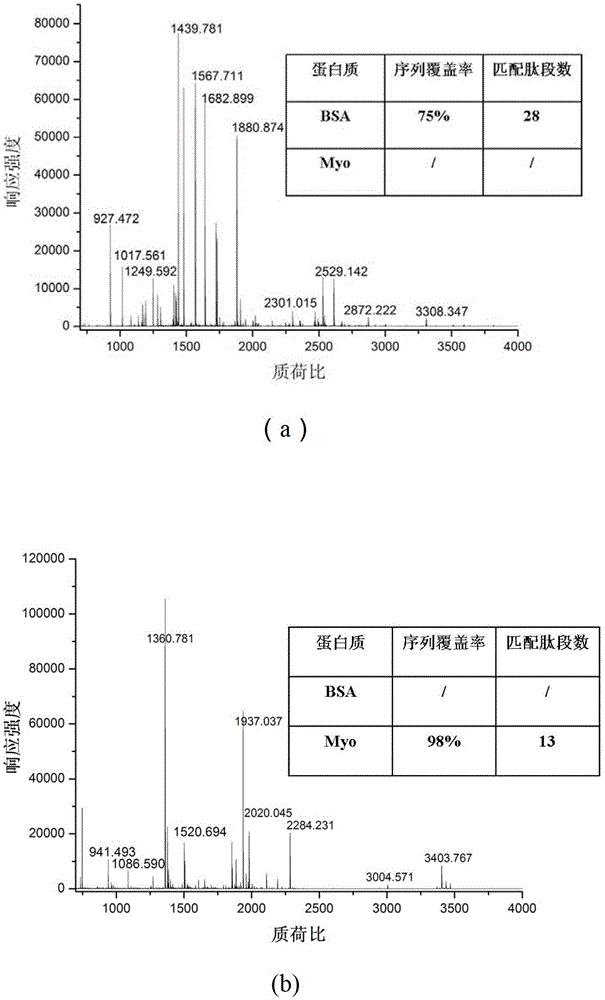
[0031] Evaluation of low-residue hybrid silica monolithic matrix enzyme reactor: 0.1mg / mL BSA and 0.05mg / mL myoglobin (Myo) were continuously passed into the immobilized enzyme reactor at 1μL / min (0.25mm i.d×50mm) In the process, the components were collected and detected by MALDI-TOF MS. After searching the database, the sequence coverage of BSA was 75% (such as figure 2 a), the sequence coverage of myoglobin is 98% (eg figure 2 b).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com