Application of ruthenium pyridine (II) complexes as antitumor drugs
A technology of ruthenium pyridine complexes, which is applied in the application field of ruthenium pyridine complexes as anti-tumor drugs, and can solve problems such as unreported and lack of research work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1 The microwave-assisted synthesis of compound I
[0030] To a 30 mL microwave Pyrex reaction tube add: cis -[Ru(bpy) 2 Cl 2 ]·2H 2 O (105 mg, 0.2 mmol), p -BrPIP (compound name is 2-(4-bromophenol)-1H-imidazol[4, 5f ][1,10]phenanthroline) (113 mg, 0.3 mmol), 15 mL ethanol. Stir for 10 min under nitrogen protection, and react at 130 °C for 20 min with microwave assistance. After the reaction was completed, spin-dried under reduced pressure to obtain a red solid, which was dried in a vacuum desiccator to obtain an orange-yellow solid. The crude product was dissolved in acetonitrile, passed through a 200-300 mesh neutral alumina column, and the main red component was washed with acetonitrile, and the solvent was spin-dried under reduced pressure to obtain a brown-red solid with a yield of 85.7%. ESI-MS (in CH 3 CN, m / z ): 789.2 ([M-H] + , calculated value: 788.1); 1 H NMR (in DMSO-d 6 , δ / ppm) 9.06 (d, J =8.3 Hz, H c , 2H), 8.86 (d, J =8.1 Hz...
Embodiment 2
[0031] Example 2 The microwave-assisted synthesis of compound II
[0032] by cis -[Ru(phen) 2 Cl 2 ]·2H 2 O alternative cis -[Ru(bpy) 2 Cl 2 ]·2H 2 O, synthetic method is basically the same as embodiment 1 [Ru (bpy) 2 ( p -BrPIP)] (ClO 4 ) 2 The synthesis of brown-red solid was obtained, and the yield was 87.9%. ESI-MS (in CH 3 CN, m / z ): 837.3 ([M+H] + , calculated value: 836.2); 1 H NMR (in DMSO-d 6 , δ / ppm) 9.03 (d, J =8.3, H c , 2H), 8.76 (d, J =8.3, H 4, 7 , 4H), 8.38 (s, H 5,6 , 4H), 8.29–8.20 (d, J =8.4, H j , 2H), 8.12 (d, J =5.3, 1.2 Hz, H 2’ , 2H), 8.07 (d, J =5.2, 1.2 Hz, H 2 , 2H), 7.99 (d, J =5.3, 1.2 Hz, H a , 2H), 7.85 (d, J =8.6 Hz, H i , 2H), 7.79 (t, J =6.6 Hz, H b , 2H), 7.76 (t, J =6.6 Hz, H 3 , 4H); 13 C NMR (101 MHz, DMSO-d 6 ) δ 152.65 (d, J =12.9 Hz), 150.13 (s), 147.17 (d, J =9.0 Hz), 145.38 (s), 136.87 (s), 136.65 (s), 132.16 (s), 130.41 (s), 128.40 (s), 128.09 (s), 126.29 (s), 126.07 (s), 123.42(s).
Embodiment 3
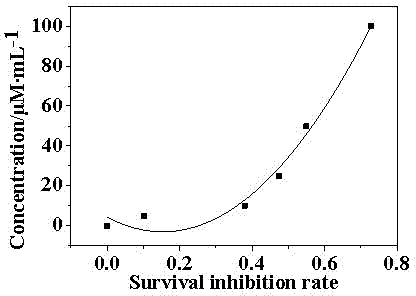
[0033] Example 3 Compound I was tested for growth inhibition of highly metastatic human breast cancer cell line (MDA-MB-231)
[0034] Will MDA-MB-231 Cells were inoculated at a certain density in 96-well plates in RPMI1640 medium (containing 0.1% penicillin, 0.1% streptomycin, and 1% glutamine) containing 10% calf serum, 5% CO 2 After culturing at 37°C for 24 h, replace with fresh medium with different concentrations of Compound I (1 μg / mL working solution prepared with PBS, diluted with medium when used) and continue culturing for 48 h; then each well Add 50 μL of pre-cooled 50% trichloroacetic acid (TCA, the final concentration is 10%), let stand for 5 min, wash with distilled water 5 times, air dry, add 100 μL of MTT staining solution, dye for 10 min, 1% acetic acid solution The cells were washed 4 times to remove unbound dye, air-dried, and finally 150 μL of 10 mmol / L Tris solution was added, and after thorough mixing, the OD value was measured with a microplate reader...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com