Binuclear ion-type phosphorescence iridium complex, preparation method and application thereof
An iridium complex, ionic technology is applied to a class of dual-nuclear ionic phosphorescent iridium complexes and their preparation and application fields, which can solve the problems of limited fluorescence characteristics, difficulty in completely eliminating background noise, and reduced fluorescence imaging sensitivity. Low toxicity, avoids photodamage and photobleaching problems, and achieves the effect of two-photon excitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
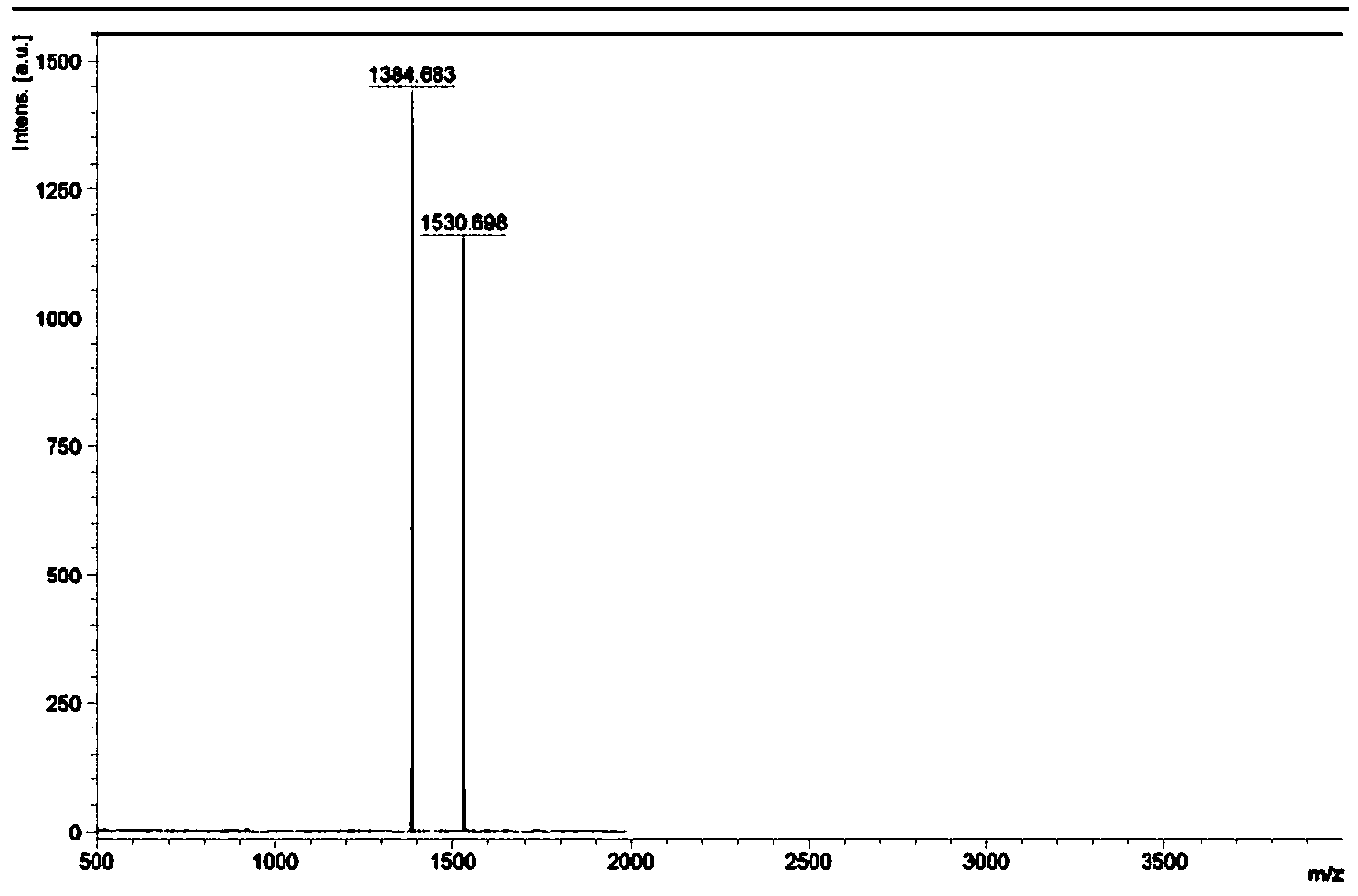
[0037] Example 1: [(ppy) 2 Ir(tpphz)Ir(ppy) 2 ] 2+ (PF 6 - ) 2 (Ir1) Preparation
[0038]
[0039] 1,10-phenanthroline-5,6-dione: In a 250mL three-necked flask, add 1,10-phenanthroline (5g, 27.7mmol) and potassium bromide (25g, 16.8mmol), and mix well. Slowly add 100mL concentrated sulfuric acid and 50mL concentrated nitric acid at 0°C, slowly raise the temperature to 70-80°C, and reflux for 1-4 hours (absorb the generated tail gas with sodium hydroxide solution). After the reaction was completed and the tail gas was removed, the system was transferred to a 500mL beaker, and solid sodium hydroxide was added to neutralize the excess acid. Observed with pH test paper, when neutralized to neutral, a large amount of flocculent precipitates are produced in the solution. After filtration, 1,10-phenanthroline-5,6-dione was obtained as a light yellow solid, which was weighed after drying, and the yield was 72% to 79%.
[0040]
[0041] tpphz: Add 1,10-phenanthroline-5,6-...
Embodiment 2
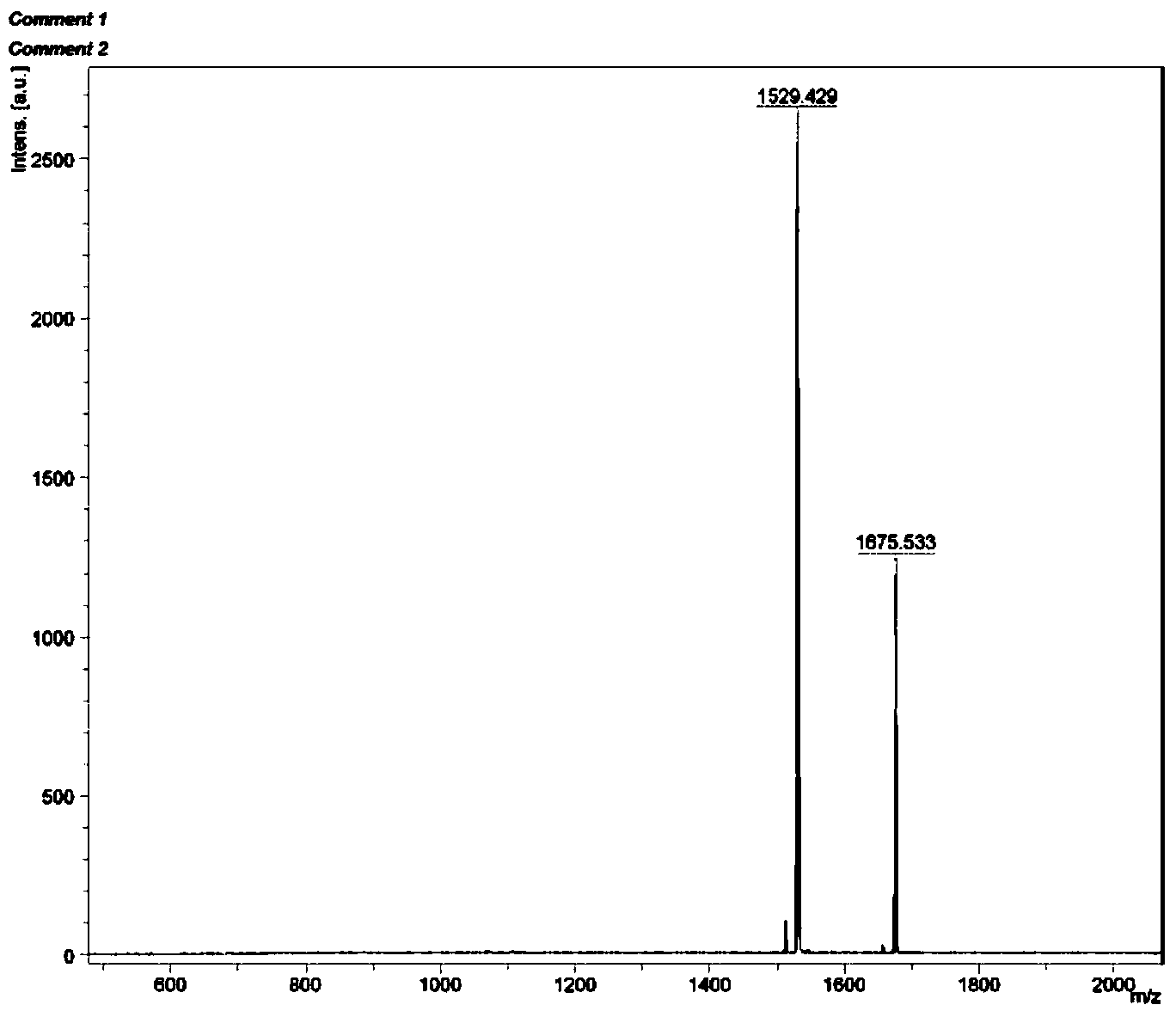
[0048] Example 2: [(dfppy) 2 Ir(tpphz)Ir(dfppy) 2 ] 2+ (PF 6 - ) 2 (Ir2) Preparation
[0049]
[0050] 1,10-phenanthroline-5,6-dione: In a 250mL three-necked flask, add 1,10-phenanthroline (5g, 27.7mmol) and potassium bromide (25g, 16.8mmol), and mix well. Slowly add 100mL concentrated sulfuric acid and 50mL concentrated nitric acid at 0°C, slowly raise the temperature to 70-80°C, and reflux for 1-4 hours (absorb the generated tail gas with sodium hydroxide solution). After the reaction was completed and the tail gas was removed, the system was transferred to a 500mL beaker, and solid sodium hydroxide was added to neutralize the excess acid. Observed with pH test paper, when neutralized to neutral, a large amount of flocculent precipitates are produced in the solution. After filtration, 1,10-phenanthroline-5,6-dione was obtained as a light yellow solid, which was weighed after drying, and the yield was 72% to 79%.
[0051]
[0052] tpphz: Add 1,10-phenanthroline-...
Embodiment 3
[0059] Embodiment 3: [(ppy) 2 Ir(tpphz)Ir(ppy) 2 ] 2+ (Cl - ) 2 Preparation of (Ir5)
[0060]
[0061] 1,10-phenanthroline-5,6-dione: In a 250mL three-necked flask, add 1,10-phenanthroline (5g, 27.7mmol) and potassium bromide (25g, 16.8mmol), and mix well. Slowly add 100mL concentrated sulfuric acid and 50mL concentrated nitric acid at 0°C, slowly raise the temperature to 70-80°C, and reflux for 1-4 hours (absorb the generated tail gas with sodium hydroxide solution). After the reaction was completed and the tail gas was removed, the system was transferred to a 500mL beaker, and solid sodium hydroxide was added to neutralize the excess acid. Observed with pH test paper, when neutralized to neutral, a large amount of flocculent precipitates are produced in the solution. After filtration, 1,10-phenanthroline-5,6-dione was obtained as a light yellow solid, which was weighed after drying, and the yield was 72% to 79%.
[0062]
[0063] tpphz: Add 1,10-phenanthroline-5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com