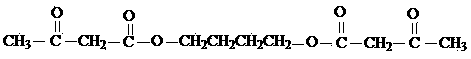Preparing method for organic intermediate diacetoacetic acid (1,4-butanediol) ester
A technology of diacetoacetic acid and butanediol, applied in the directions of organic chemistry, ketene/polyketene preparation, etc., can solve the problems of difficult transesterification reaction, low product yield and the like, and achieves low cost, high product yield, The effect of less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] In a four-necked reaction flask equipped with an electric stirrer, a reflux condenser and a thermometer, add 18g of 1,4-butanediol, 18.5g of diketene, 0.5g of a polymerization inhibitor, 0.3g of a catalyst, and 30g of a solvent , install a reflux condenser and a thermometer, start the agitator, and start timing when the temperature rises to 85°C, and the reaction time is 8-14h. After the reaction is completed, the reactant is alkali-washed with 10-15% (mass) lye, the water phase is separated, and atmospheric distillation is carried out to reclaim the solvent and unreacted raw materials, and then the remaining reactant is subjected to vacuum distillation, namely A colorless and transparent liquid diacetoacetate (1,4-butanediol) ester product was obtained, and the yield of the product reached 91.18%.
Embodiment 2
[0019] In a four-necked reaction flask equipped with an electric stirrer, a reflux condenser and a thermometer, add 18g of 1,4-butanediol, 21g of diketene, 0.5g of inhibitor, 0.3g of catalyst, 30g of solvent, Install a reflux condenser and a thermometer, start the stirrer, and start timing when the temperature rises to 85°C, and the reaction time is 8-14h. After the reaction is completed, the reactant is alkali-washed with 10-15% (mass) lye, the water phase is separated, and atmospheric distillation is carried out to reclaim the solvent and unreacted raw materials, and then the remaining reactant is subjected to vacuum distillation, namely A colorless and transparent liquid diacetoacetate (1,4-butanediol) ester product was obtained, and the yield of the product reached 91.49%.
Embodiment 3
[0021] In the four-necked reaction flask equipped with electric stirrer, reflux condenser and thermometer, add 18g of 1,4-butanediol, 25.2g of diketene, 0.8g of inhibitor, 0.6g of catalyst, 30g of solvent , install a reflux condenser and a thermometer, start the agitator, and start timing when the temperature rises to 85°C, and the reaction time is 8-14h. After the reaction is completed, the reactant is alkali-washed with 10-15% (mass) lye, the water phase is separated, and atmospheric distillation is carried out to reclaim the solvent and unreacted raw materials, and then the remaining reactant is subjected to vacuum distillation, namely A colorless and transparent liquid (1,4-butanediol) diacetoacetate product was obtained, and the yield of the product reached 91.97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com