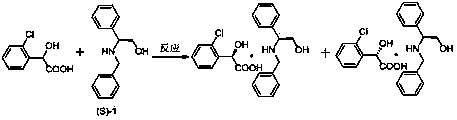
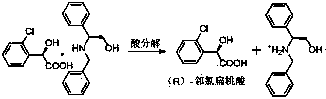
Preparation method of novel chiral resolving agent and (R)-chloromandelic acid
A chiral separation technology of o-chloromandelic acid, applied in the field of medicine and chemical industry, can solve the problems of difficult industrial production of separation efficiency, and achieve the effects of environmental protection and safe production, easy separation and recovery, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The chiral resolving agent S-(1) of this embodiment is R in general formula I 1 =Cl, the substitution position is ortho, R 2 =H, the specific structure of (S)-1 in this embodiment is as follows:
[0048]
[0049] A preparation method of (R)-o-chloromandelic acid, the specific steps are as follows:
[0050] Fill the reaction vessel with nitrogen in advance for replacement, weigh 18.6g (0.1mol) of racemic (RS)-o-chloromandelic acid and completely dissolve it in 69g (1.5mol) of absolute ethanol, stir and cool down to 15°C, slowly Slowly add the same amount of (S)-1, after the addition is complete, there will be a large amount of white precipitate in the solution, heat up to 80°C, keep warm and continue to stir for 0.5 hours, keep stirring and naturally cool down to 20°C, place Filter after 10 hours, wash the filtered white needle-like solid with absolute ethanol that has been cooled to 0°C in advance, and dry it in a vacuum oven at 60°C to constant weight. The white n...
Embodiment 2
[0052] The chiral resolving agent S-(1) of this embodiment is R in general formula I 1 =Cl, the substitution position is para, R 2 =NO 2 , the substitution position is the para position, and the specific structure of (S)-1 is as follows:
[0053]
[0054] A preparation method of (R)-o-chloromandelic acid, the specific steps are as follows:
[0055] Fill the reaction vessel with nitrogen in advance for replacement, weigh 558g (3mol) of racemic (RS)-o-chloromandelic acid and completely dissolve it in 1334g (18mol) of tert-butanol, stir and cool down to 15°C, and slowly add 644g (2.1mol) of (S)-1, from the addition of (S)-1, there is a white precipitate in the solution, heat up to 120°C, keep warm and continue to stir for 2 hours, when the temperature is lowered to 80°C, add a small amount of ( R)-o-chloromandelic acid·(S)-1 diastereoisomeric salt as seed crystal, keep stirring and cool down naturally to lower the temperature to 18°C, let stand for 5 hours and then filter, ...
Embodiment 3
[0058] The chiral resolving agent S-(1) of this embodiment is R in general formula I 1 = H, R 2 =NO 2 , the substitution position is the para position, and the specific structure of (S)-1 is as follows:
[0059]
[0060] A preparation method of (R)-o-chloromandelic acid, the specific steps are as follows:
[0061] Fill the reaction vessel with argon in advance for replacement, weigh 186g (1mol) of racemic (RS)-o-chloromandelic acid and completely dissolve it in 300g (5mol) of isopropanol, stir and cool down to 15°C, slowly Add 109g (0.4mol) (S)-1, a large amount of white precipitates are produced in the solution during the addition process, heat up to 70°C, keep warm and continue to stir for 1 hour, keep stirring and naturally cool down to 10°C, place for 12 After 1 hour, filter, and the filtered white needle-shaped solid is washed with isopropanol that has been cooled to 0°C in advance, and dried in a vacuum oven to constant weight. The obtained white needle-shaped soli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com