Application of procyanidin B2 in medicaments
A proanthocyanidin and drug technology, applied in the field of proanthocyanidin B2 in medicine, to achieve the effects of avoiding damage, enhancing curative effect, and inhibiting and protecting bone marrow DNA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] Preparation of reference substance solution: Accurately weigh 50 mg of gallic acid reference substance, put it in a 100ml brown measuring bottle, add water to dissolve and dilute to the mark, accurately measure 5ml, put it in a 50ml brown measuring bottle, dilute with water to the mark, shake well, that is Obtained (every 1ml contains gallic acid 0.05mg).
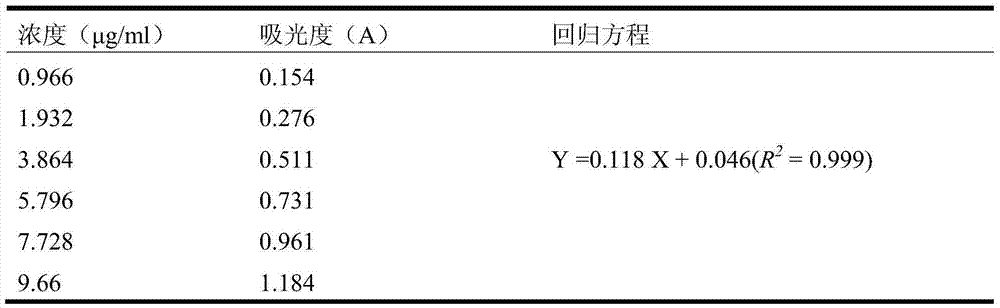
[0075] Preparation of the standard curve: Accurately measure 0.5ml, 1.0ml, 2.0ml, 3.0ml, 4.0ml, 5.0ml of the reference solution, put them in 25ml brown measuring bottles, add 1ml of phosphomolybdotungstic acid test solution, and then add water respectively 11. 50ml, 11ml, 10ml, 9ml, 8ml, 7ml, dilute to the mark with 29% sodium carbonate solution, shake well, let stand for 30 minutes and use the corresponding reagent as a blank, according to ultraviolet-visible light photometry (Appendix V A), at 760nm Measure the absorbance at the wavelength, draw the standard curve with the absorbance as the ordinate and the concent...
Embodiment 1
[0124] Embodiment 1: the preparation of Burnet tannin extract of the present invention
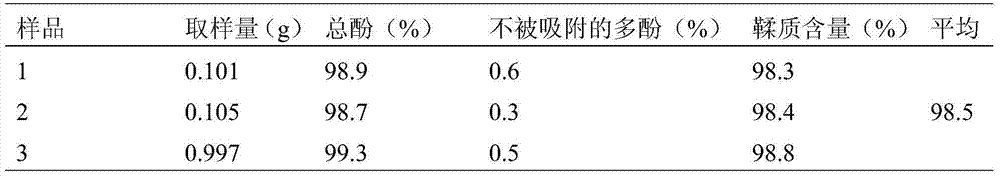
[0125] Grind Burnet medicinal material into coarse powder, flash extract 2 times with 70% acetone, 2 minutes each time, 10 times the amount of solvent for the first time, 8 times the amount for the second time, combine the filtrate, concentrate under reduced pressure, diethyl ether and concentrated solution 1 : 1 volume ratio extraction and degreasing until the ether layer is colorless, then extract and enrich the total phenols with ethyl acetate and mother liquor 1:1 volume ratio 6 times, combine ethyl acetate, concentrate under reduced pressure at 45°C to an appropriate amount, and reduce pressure at 45°C Dry and serve.
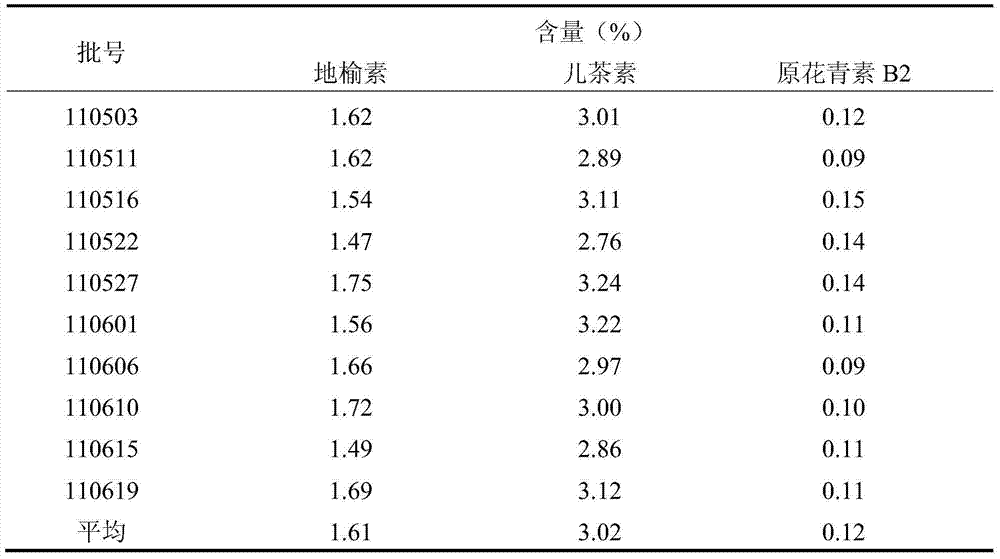
[0126] The results of content determination are: the extract contains 62% tannin, 1.32% burnet, 2.61% catechin, 20.08% proanthocyanidin B, 6.2% burnet saponin, 3.7% burnet Ⅰ: 3.7%, all by weight percentage content.
Embodiment 2
[0127] Embodiment 2: the preparation of Burnet tannin extract of the present invention
[0128] Grind Burnet medicinal material into coarse powder, reflux and extract with 70% ethanol for 3 times, each time for 2 hours, the amount of solvent is 10 times for the first time, 8 times for the second time, 6 times for the third time, combine the filtrates, and concentrate under reduced pressure , diethyl ether and concentrated solution at a volume ratio of 1:1 to extract and degrease until the ether layer is colorless, then extract and enrich total phenols with ethyl acetate and mother liquor at a volume ratio of 1:1 for 6 times, combine ethyl acetate, and concentrate under reduced pressure at 45°C to Appropriate amount, dried under reduced pressure at 45°C.
[0129] The results of content determination are: the extract contains 65% tannin, 1.41% burnet, 2.65% catechin, 20.09% proanthocyanidin B, 5.4% burnet saponin, 3.1% burnet Ⅰ: 3.1%, all by weight percentage content.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com