A preparation for improving oral bioavailability of risedronate sodium and a preparing method thereof
A technology of risedronate sodium and availability, applied in the field of medicine, to achieve the effect of improving dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
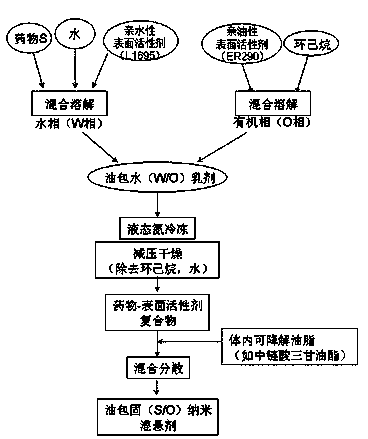
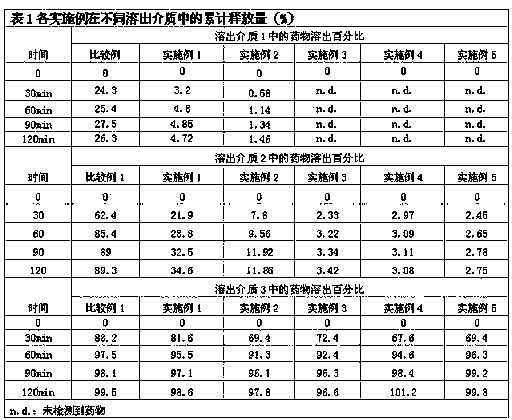
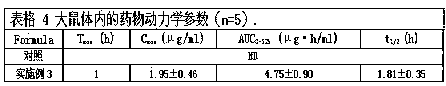
[0020] Example 1 Weigh 0.201 g of risedronate sodium hydrate (containing 0.175 g of risedronate sodium) and add 0.02 g of sucrose monolaurate (trade name: L1695; Japan Mitsubishi Chemical Foods Co., Ltd.) into 10 mL of purified water to dissolve and set aside. Another 0.173 g of sucrose sinapinate (trade name: ER290; Japan Mitsubishi Chemical Foods Co., Ltd.) was weighed in 20 mL of cyclohexane. The above two-phase solution was placed in a 50 mL eggplant-shaped bottle, and a water-in-oil (W / O) emulsion was prepared using a high-speed shear (23000 rpm, 5 min). After being frozen with liquid nitrogen, the mixture is placed in a freeze dryer for 24 hours to freeze-dry to remove the cyclohexane and the water phase, and then the compound containing the drug and the surfactant is obtained. Add 9.65 g of medium-chain fatty acid triglycerides (MCT). After being stirred uniformly, a solid-in-oil suspension containing risedronate sodium was obtained. By weight, the drug content is 1...
Embodiment 2
[0021] Example 2 Weigh 0.201 g of risedronate sodium hydrate (containing 0.175 g of risedronate sodium) and add it into 10 mL of purified water containing 0.02 g of L1695 to dissolve it for later use, and weigh 0.505 g of ER290 in 20 mL of cyclohexane. Referring to the preparation method of Example 1, a compound containing a drug and a surfactant was obtained. Add 9.3 g MCT. By weight, the drug content is 1.75%, the surfactant accounts for 5.25% (the ratio of drug to surfactant is 1:3), and the external oil phase accounts for 93%.
Embodiment 3
[0022] Example 3 Weigh 0.201 g of risedronate sodium hydrate (containing 0.175 g of risedronate sodium) and add 0.02 g of sucrose monolaurate in 10 mL of purified water to dissolve it for later use; another weigh 0.855 g of ER290 and dissolve it in 20 mL of cyclohexane alkane. Referring to the preparation method of Example 1, the complex of the drug and the surfactant was obtained. Add 8.95 g of medium-chain fatty acid triglycerides and disperse evenly to obtain a solid-in-oil suspension containing risedronate sodium. By weight, the drug accounts for 1.75%. Surfactant accounts for 8.75% (the ratio of drug to surfactant is 1:5), and the external oil phase accounts for 89.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com