Preparation method of block-type high polymerization degree macromonomer methyl allyl polyoxyethylene polyoxypropylene ether
A technology of methyl alkenyl polyoxyethylene polyoxypropylene ether and high degree of polymerization, which is applied in the field of polyether synthesis in organic chemistry, and can solve the problem that the performance cannot meet the polymerization requirements of polycarboxylate water reducers, the molecular weight is difficult to increase, and the molecular weight Wide distribution and other issues, to achieve the effect of improving the double bond retention rate, high degree of polymerization, and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
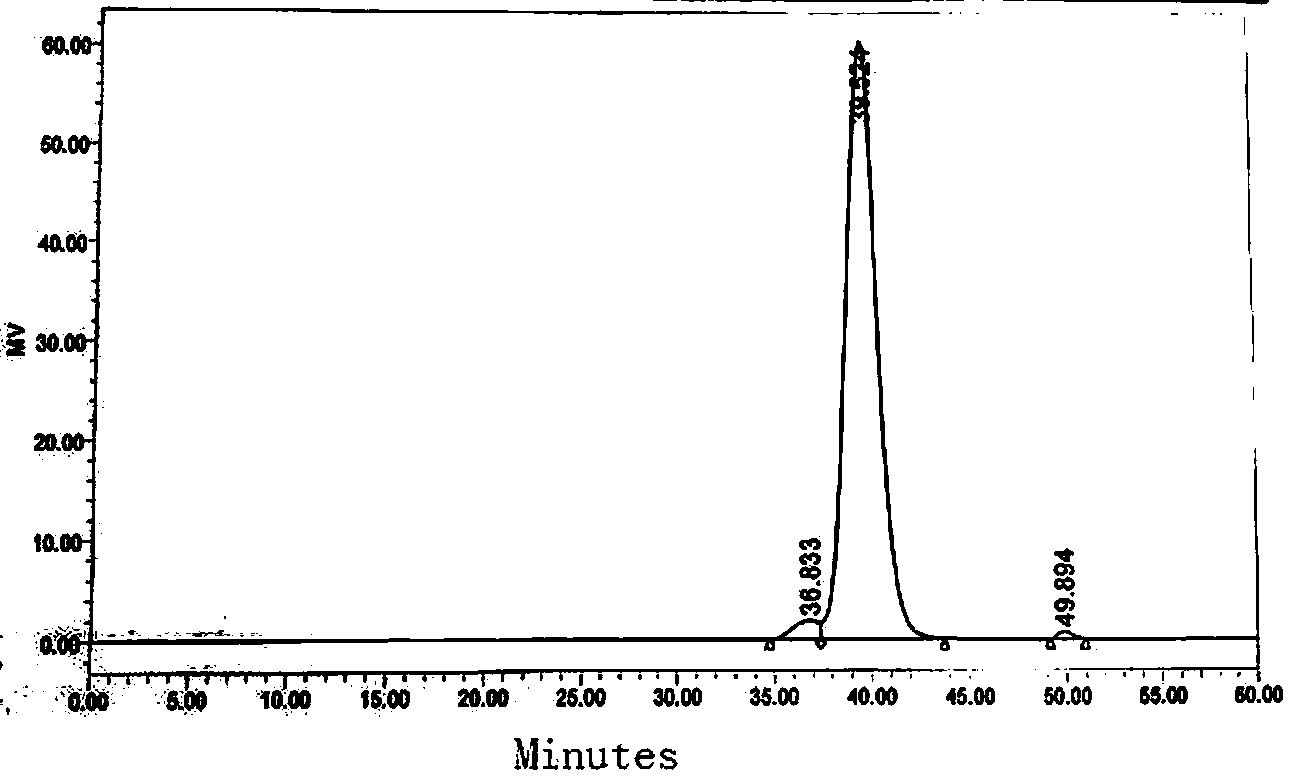
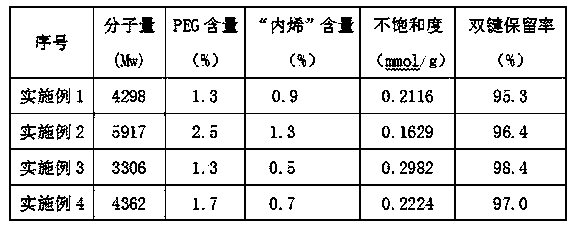
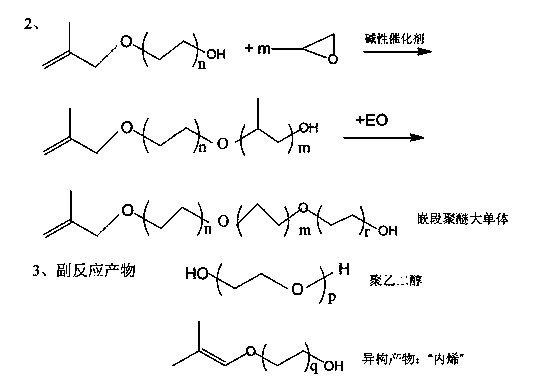
Image
Examples
Embodiment 1
[0033] Preparation of 2-methyl-2-propenyl-1-ol polyoxyethylene (87.5) polyoxypropylene (10) ether
[0034] (1) Add 3780g of 2-methyl-2-propenyl-1-ol (52.5mol) and 50g of beaded sodium hydroxide into the external circulation ethoxylation reactor, dehydrate in vacuum for 1 hour, and replace with nitrogen three times, Turn on the circulation pump, gradually increase the temperature of the kettle to 100°C, and feed 46,200g of ethylene oxide. After the feed is complete, heat preservation and aging reaction for 0.5 hours, and then cool down to 60°C to obtain methyl alkenyl alcohol polyoxyethylene ether oligomerization substance (referred to as TPEG-20B).
[0035] (2) Add 10,000 g of the oligomer (TPEG-20B) obtained in step (1) and 52.3 g of sodium methoxide into the reactor, replace it with nitrogen three times, turn on the circulation pump, gradually raise the temperature to 100 ° C, and vacuum dealcoholize for 1 hour; Pass into 6090g of propylene oxide then, continue to insulate ...
Embodiment 2
[0038] Preparation of 2-methyl-2-propenyl-1-ol polyoxyethylene (110) polyoxypropylene (20) ether
[0039] (1) Add 2590g of 2-methyl-2-propenyl-1-ol (35.97mol) and 25g of sodium hydride into the external circulation ethoxylation reactor, vacuum dehydrate for 1 hour, replace with nitrogen three times, and turn on the circulation pump , gradually raise the temperature of the kettle to 80°C, feed 47,483g of ethylene oxide, and then heat-preserve and ripen the reaction for 1 hour after passing through completely, then cool down to 60°C, and send the intermediate (TPEG-30B for short) to The intermediate storage tank is protected with nitrogen gas.
[0040] (2) Add 11620g of the oligomer (TPEG-30B) obtained in step (1) and 285.8g of sodium hydroxide into a 60L external circulation ethoxylation reactor, replace it with nitrogen three times, turn on the circulation pump, and gradually raise the temperature To 130°C, vacuum dehydration for 0.5 hours; then 9683g of propylene oxide was p...
Embodiment 3
[0043] Preparation of 3-methyl-3-butenyl-1-ol polyoxyethylene (60) polyoxypropylene (10) ether
[0044] (1) Add 3050g 3-methyl-3-butenyl-1-ol (3-methyl-3-buten-1-ol, 35.46mol) and 74.6g methanol to the external circulation ethoxylation reactor Sodium, dealcoholized in vacuum for 1 hour, replaced with nitrogen three times, turned on the circulation pump, gradually increased the temperature of the kettle to 40°C, dealcoholized in vacuum for 1 hour, controlled the vacuum degree at -0.065MPa, and then began to introduce 15602g of ethylene oxide , After the feed is complete, heat preservation and aging reaction for 0.5 hours, the temperature is lowered to 60 ° C, and the intermediate (abbreviated as TPEG-10A) is sent to an intermediate storage tank with nitrogen pressure and protected with nitrogen.
[0045] (2) Add 5839g of the oligomer (TPEG-10A) obtained in step (1) and 264.6g of sodium methoxide into a 60L external circulation ethoxylation reactor, replace it with nitrogen thre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
| Hydroxyl value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com