Method for preparing 16-dehydrogenated pregnane dehydropregnenolone acetate compound by using photosensitized oxidation of blue LED (light-emitting diode) light source
A diketene alcohol acetic acid, photosensitive oxidation technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of water resource waste cost, energy waste, cost occupied by filter systems, etc., to save electric energy and eliminate environmental pollution problems Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
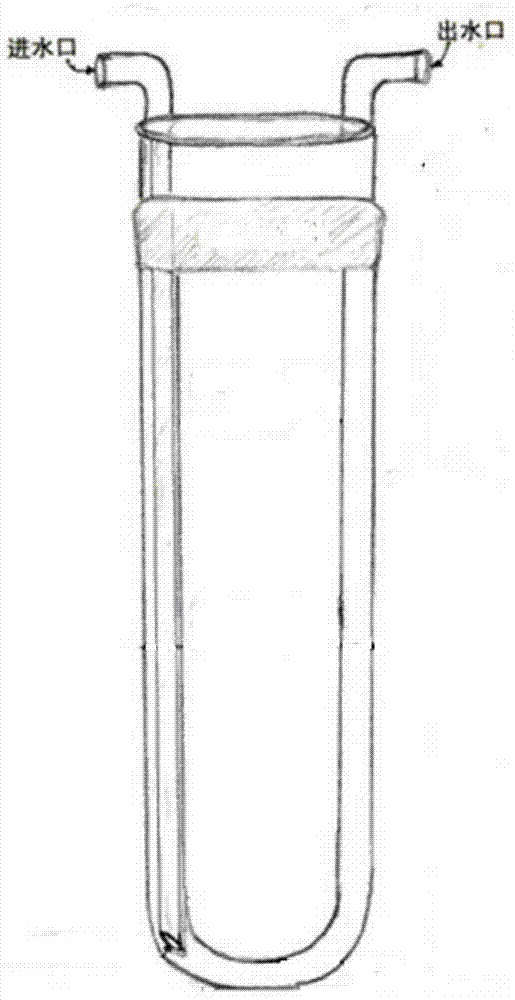
[0034] The used internal immersion bubbling photochemical reactor is composed of a container with an air inlet at the bottom, a sink and a 10W blue LED lamp with an emission wavelength greater than 380nm.
[0035] Such as figure 1 As shown, the sinker made of glass capable of filtering light below 380nm is a U-shaped interlayer container with a cooling water inlet and outlet, and the sinker is installed in the container; the blue A colored LED light is placed in the inner cavity of the sink.
[0036] Utilize the above-mentioned internal immersion bubbling photochemical reactor to oxidatively degrade pseudodiosgenin diacetate into 3β-hydroxy-pregna-5(6),16(17)-dien-20-one acetate:
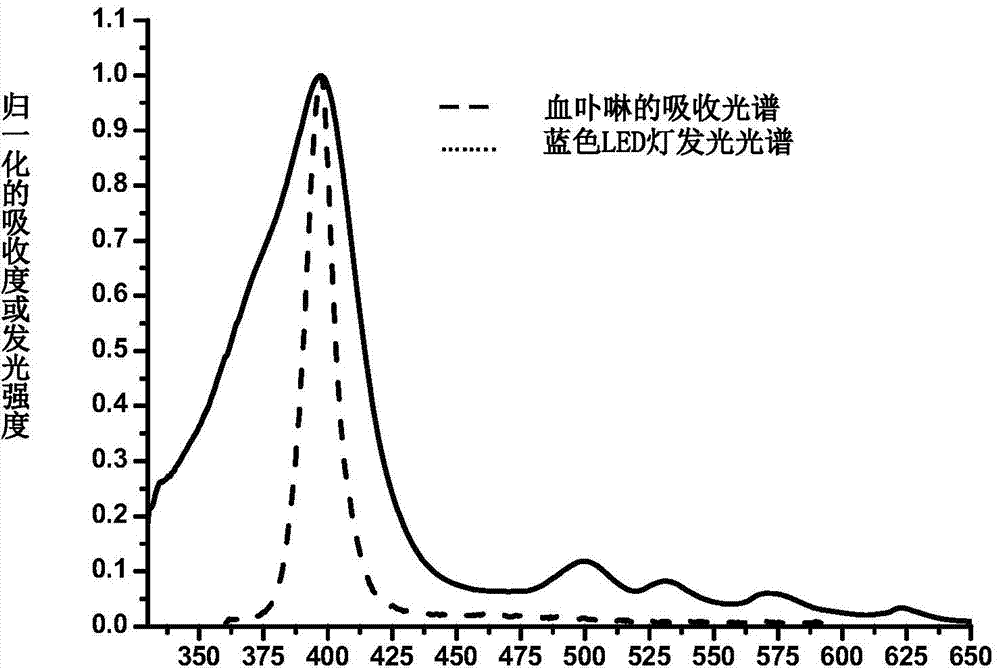
[0037] At room temperature (such as 25°C), in a 500mL round-bottomed flask, dissolve 10g of pseudodiosgenin diacetate, 37mg of hematoporphyrin, 5.5mL of triethylamine and 3.7mL of acetate in 330mL of In 2-methyltetrahydrofuran, stir and mix evenly to prepare a photochemical reaction solution. Tra...
Embodiment 2
[0045] Utilize the internal immersion bubbling photochemical reactor of Example 1, oxidative degradation pseudodiosgenin diacetate is 3β-hydroxyl-pregna-5(6),16(17)-dien-20-one acetate:
[0046] At room temperature (such as 25°C), in a 500mL round-bottomed flask, dissolve 10g of pseudodiosgenin diacetate, 37mg of hematoporphyrin, 5.5mL of triethylamine and 3.7mL of acetate in 330mL of ethyl acetate, stir and mix evenly, and prepare photochemical reaction solution. Transfer the photochemical reaction liquid to the container of the inner immersion bubbling photochemical reactor, and pass oxygen into the photochemical reaction liquid from the bottom of the container for bubbling, adjust the flow of oxygen to make the bubbles uniform; open the inner immersion bubbling photochemical reactor The cooling water valve of the reactor is opened, and the blue LED light of 10W installed in the sinking trap in the bubbling photoreactor is turned on, and the photosensitive oxidation reactio...
Embodiment 3
[0052] Using the internal immersion bubbling photochemical reactor of Example 1, the method for oxidatively degrading pseudosagagenin diacetate into 5α-pregnene-12,20-diketone-3β-hydroxyacetate is basically the same as that of Example 1. Same, just change 2-methyltetrahydrofuran into dioxane, the raw material pseudodiosgenin diacetate is changed into pseudosacegenin diacetate, and utilize high pressure liquid chromatography (HPLC) to monitor the reaction process (HPLC detection condition Same as Example 1). At the end of the photosensitive oxidation reaction, the conversion rate of the raw material pseudosacetin diacetate was 98.8%.
[0053] The obtained light brown crystalline 5α-pregnene-12,20-diketone-3β-hydroxyacetate was 6.4 g (purity: 96.8%), and the yield was 74.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com