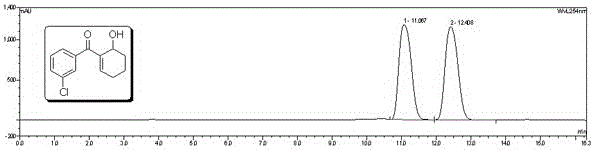
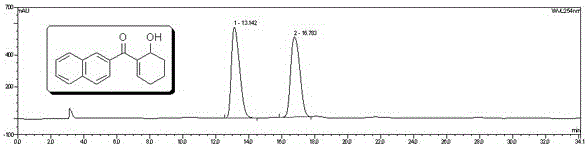
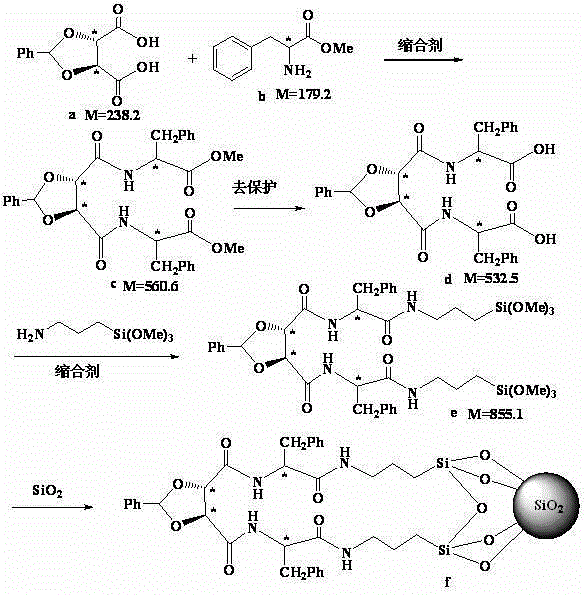
Bidentate Chiral Chromatographic Silanes and Chiral Stationary Phases Containing Tartrate Skeleton
A chiral and silane technology, applied in the field of chromatographic separation materials, can solve the problems of weak force of coating-type stationary phase, etc., achieve good chiral separation effect, simple synthesis method, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Source of raw material 1
[0026] Dimethyl (+)-2,2-dimethyl-1,3-dioxolane-4,5-dicarboxylate (M = 190) Alkaline hydrolysis to obtain (+)-2,2-dimethyl-1,3-dioxolane-4,5-dicarboxylic acid dicarboxylic acid (M = 178).
[0027] Silane preparation
[0028] (1) Preparation of Intermediate 3
[0029] in N 2 Under protection, the raw material of 10.3g (50mmol) ( 1 ), 19.8g (110mmol) of raw material ( 2 ) was dissolved in 100ml of dichloromethane, continued stirring for 5-10 minutes, added 110mmol HBTU, 110mmol HOBT, 100ml DIEA, and stirred at room temperature for 2h. The reaction was detected by TLC point plate until the reactant ( 1 )disappear. The dichloromethane was removed by rotary evaporation, and the concentrate was added to refrigerated ether for precipitation, washed three times with refrigerated ether, and the intermediate ( 3 ). MS:m / z513.55[M+H] + . Elemental analysis: C%63.46; H%6.23; N%5.41.
[0030] (2) Preparation of Intermediate 4
[0031] ...
Embodiment 2
[0044] Silane preparation
[0045] (1) Preparation of Intermediate C
[0046] in N 2 Under protection, the raw material of 9.5g (40mmol) ( a) , 15g (84mmol) of raw material ( b) Dissolve in 100ml DMF, continue stirring for 5-10 minutes, add 84mmol HBTU, 84mmol HOBT, 50ml DIEA, and stir at room temperature for 2 hours. The reaction was detected by TLC point plate until the reactant ( a) disappear. The solvent was removed by rotary evaporation, the concentrate was precipitated by adding refrigerated ether, and washed three times with cold ether to obtain the intermediate ( c ). MS:m / z561.59[M+H] + . Elemental analysis: C%66.38; H%5.64; N%4.97.
[0047] (2) Preparation of intermediate d
[0048] Take by weighing 11.2g (20mmol) intermediate ( c ), add 100ml of 1N NaOH solution and 50ml of toluene, stir magnetically at 80°C for 2-3 hours, cool to room temperature, drop in 1N dilute hydrochloric acid to adjust the pH to neutral, evaporate to dryness under reduced pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com