High performance liquid chromatography determination method of dihydroartemisinin content
A high-performance liquid chromatography and dihydroartemisinin technology, applied in measuring devices, instruments, scientific instruments, etc., to achieve good reproducibility, stable sum of double peak areas, and improved accuracy and reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
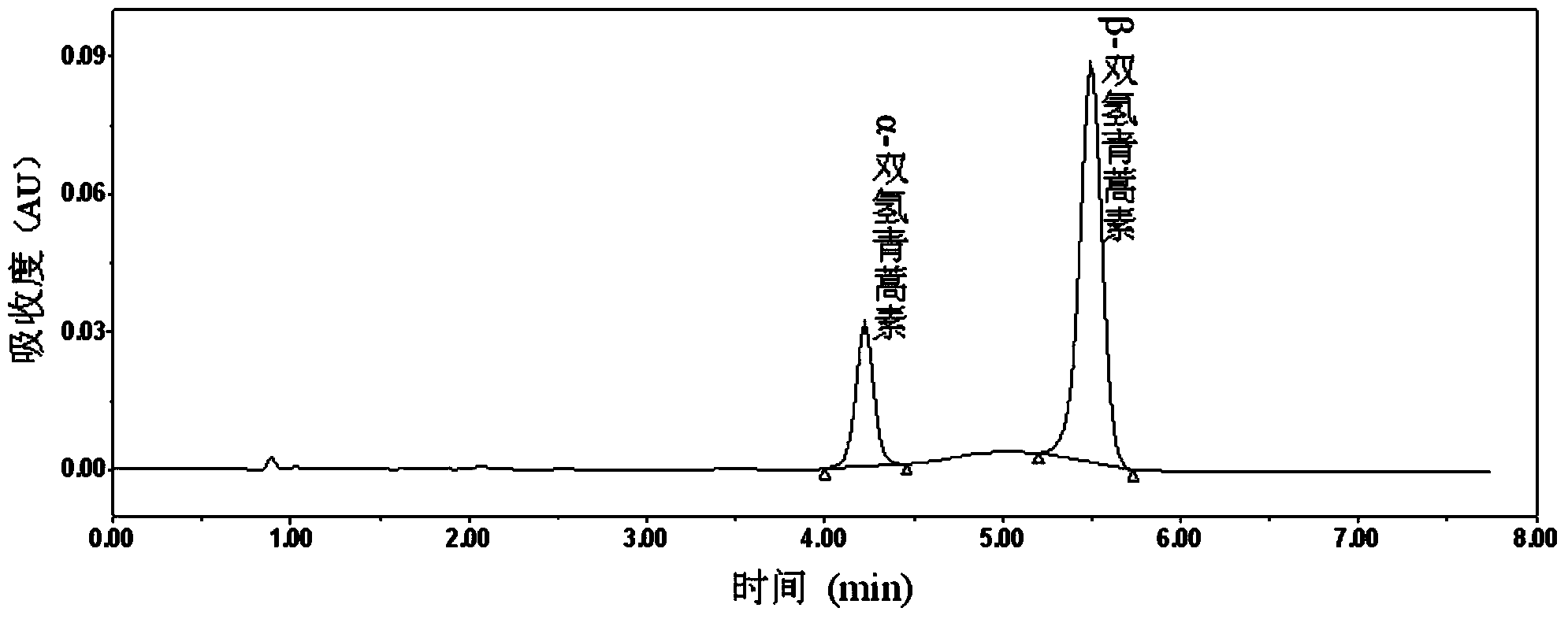
[0028] Precisely weigh 25.22mg of dihydroartemisinin reference substance, put it in a 25ml measuring bottle, add an appropriate amount of acetonitrile-water (60:40), dissolve it by ultrasonic treatment, dilute to the mark, shake well, and use it as the test solution; Inject 20 μl into the liquid chromatograph and record the chromatogram. Octadecylsilane bonded silica gel (CAPCELL PAK C18MGⅡ, 100mm×4.6mm, 3μm) was used as filler; acetonitrile-water (60︰40) was used as mobile phase; detection wavelength was 216nm; flow rate was 0.6ml / min. The chromatographic peak area of dihydroartemisinin was measured at 0min, 10min, and 20min respectively, and the high-performance liquid chromatogram is as follows Figure 1~3 shown. The chromatographic peaks of dihydroartemisinin all showed double peaks. according to Figure 1~3 Calculate the RSD% of the sum of the chromatographic peak areas and the RSD% of the ratio of the chromatographic peak areas, and the results are shown in Table 1....
Embodiment 2
[0032] Accurately weigh 40.12 mg of dihydroartemisinin reference substance, put it in a 10ml measuring bottle, add an appropriate amount of dimethyl sulfoxide, shake to dissolve, dilute to the mark, shake well, and use it as the test solution; accurately measure 5 μl of injection solution Phase chromatograph, record the chromatogram. Octadecylsilane bonded silica gel (CAPCELL PAK C18MGⅡ, 100mm×4.6mm, 3μm) was used as filler; acetonitrile-water (60︰40) was used as mobile phase; detection wavelength was 216nm; flow rate was 0.6ml / min. The chromatographic peak area of dihydroartemisinin was measured at 0min, 10min, and 20min respectively, and the high-performance liquid chromatogram is as follows Figure 4~6 shown. The chromatographic peak of dihydroartemisinin was doublet. according to Figure 4~6 Calculate the RSD% of the sum of the chromatographic peak areas and the RSD% of the ratio of the chromatographic peak areas, and the results are shown in Table 2. It can be seen ...
Embodiment 3
[0036] Accurately weigh 40.33 mg of dihydroartemisinin raw material drug sample, put it in a 10ml measuring bottle, add an appropriate amount of dimethyl sulfoxide, shake to dissolve, dilute to the mark, shake well, and use it as a test solution; accurately measure 5 μl Inject into the liquid chromatograph and record the chromatogram. Octadecylsilane bonded silica gel (EC100 / 4.6NUCLEOSIL100-3C18, 3μm, 4.6×100mm) was used as filler; acetonitrile-water (60︰40) was used as mobile phase; detection wavelength was 216nm; flow rate was 0.6ml / min. Samples were injected at 0 min, 10 min, and 20 min to measure the chromatographic peak area of dihydroartemisinin. The chromatographic peak of dihydroartemisinin was double-peaked, and the sum of the peak areas was stable. The calculated RSD% of the chromatographic peak area was 0.45 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com