Method for extracting high-purity pyrite and arsenopyrite from carlin-type gold deposit
A technology for golden pyrite and pyrite, which is applied in the field of extracting high-purity pyrite and arsenopyrite, which can solve the problems that gold-bearing minerals cannot be reached, the chemistry or physics of gold-bearing minerals cannot be correctly expressed, and research cannot be carried out.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
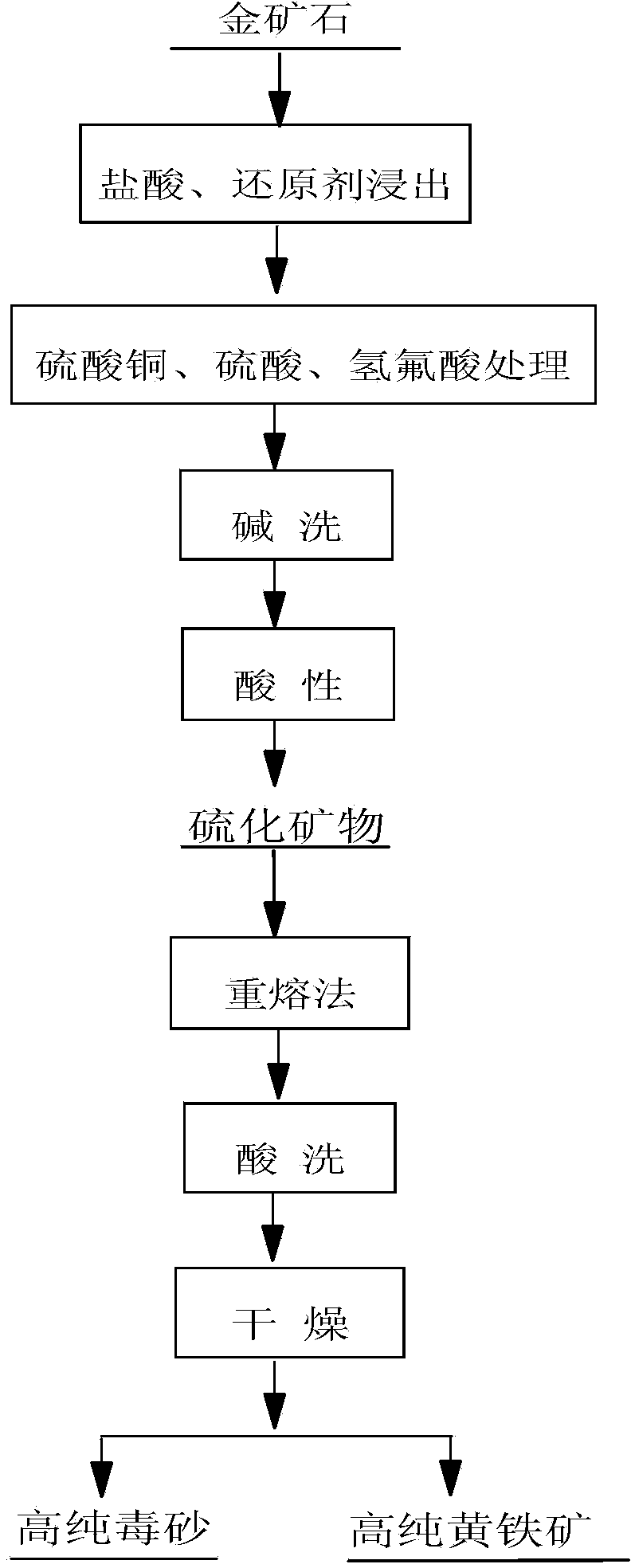
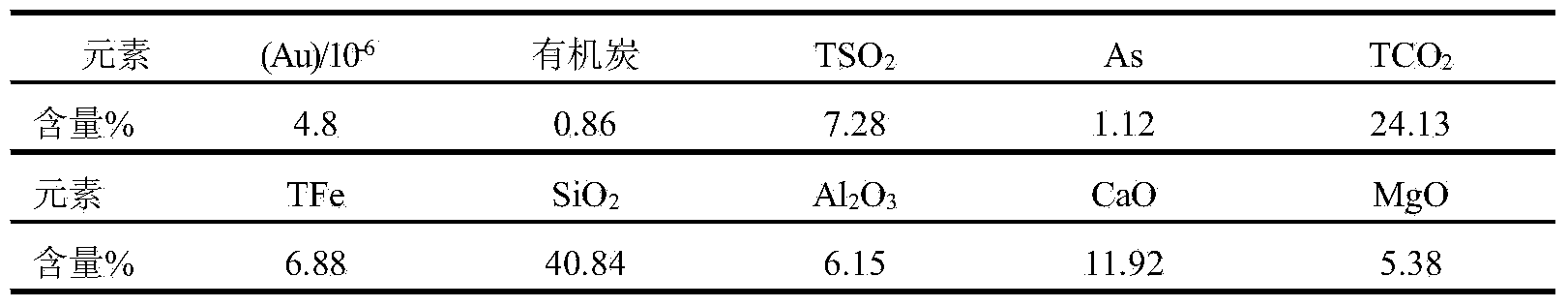
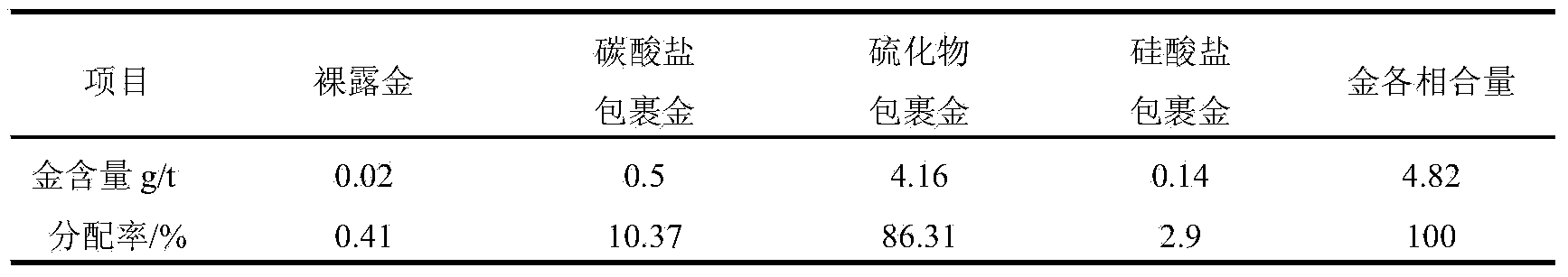
[0031] Example 1: A certain ore in Guizhou was leached with 20% hydrochloric acid and the reducing agent sodium sulfite, stirred and leached at a constant temperature of 70°C for 1 hour, filtered, washed 3 times with dilute hydrochloric acid, washed 4 times with deionized water, and dried at 65°C. Transfer the filter residue into a polytetrafluoroethylene beaker, add copper sulfate, 20% sulfuric acid and hydrofluoric acid, heat on the electric hot plate to decompose silicate minerals, add hydrofluoric acid after 1 hour, continue heating for 30 minutes after stirring, and then solid-liquid separate. When filtering, wash 5 times with water, then wash 1 time with 1% sodium hydroxide solution, keep it wet on the filter paper for about 1 hour, then wash 8 times with 5% dilute hydrochloric acid solution, and finally wash the filter residue with deionized water, Obtain sulfide minerals with a purity greater than 95%. Prepare a silver nitrate-silver iodide mixture with a melting poin...
example 2
[0032] Example 2: A certain ore in Guizhou was leached with 10% hydrochloric acid, stirring and leaching at room temperature for 1 hour, filtered, washed 3 times with dilute hydrochloric acid, then washed 4 times with deionized water, and dried at 65°C. Transfer the filter residue into a polytetrafluoroethylene beaker, add copper sulfate, 20% sulfuric acid and hydrofluoric acid, heat on the electric heating plate for 30 minutes, then add hydrofluoric acid, continue heating for 30 minutes, and then separate the solid and liquid. When filtering, wash 5 times with water, then wash 1 time with 1% sodium hydroxide solution, keep it in a wet state on the filter paper for about 30 minutes, then wash 8 times with 5% dilute hydrochloric acid solution, and finally wash the filter residue with deionized water. Obtain sulfide minerals with a purity greater than 80%. Prepare a silver nitrate-silver iodide mixture with a melting point of 280°C and a mixing specific gravity of 5.28. Use a c...
example 3
[0033]Example 3: A certain ore in Zhejiang was leached with 20% hydrochloric acid and the reducing agent sodium sulfite, stirred and leached at 70°C for 1 hour, filtered, washed 3 times with dilute hydrochloric acid, washed 4 times with deionized water, and dried at 100°C. Transfer the filter residue into a polytetrafluoroethylene beaker, add copper sulfate, 20% sulfuric acid and hydrofluoric acid, heat and decompose on the electric heating plate for 1 hour, then add hydrofluoric acid, continue heating for 1 hour, and then separate the solid and liquid. When filtering, wash 5 times with water, then wash 1 time with 1% sodium hydroxide solution, keep it wet on the filter paper for about 1.5 hours, then wash 8 times with 5% dilute hydrochloric acid solution, and finally wash the filter residue with deionized water , to obtain sulfide minerals with a purity greater than 95%. Prepare a silver nitrate-silver iodide mixture with a melting point of 280°C and a mixing specific gravity...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com