Preparing process for vildagliptin/metformin hydrochloride compound preparation
A kind of technology of metformin hydrochloride and compound preparation, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Prescription:
[0019] Element Quantity / piece Vildagliptin 50mg Metformin Hydrochloride 1000mg Hydroxypropyl Cellulose 130mg Magnesium Aluminum Silicate 6mg Magnesium stearate 12mg
[0020] production method:
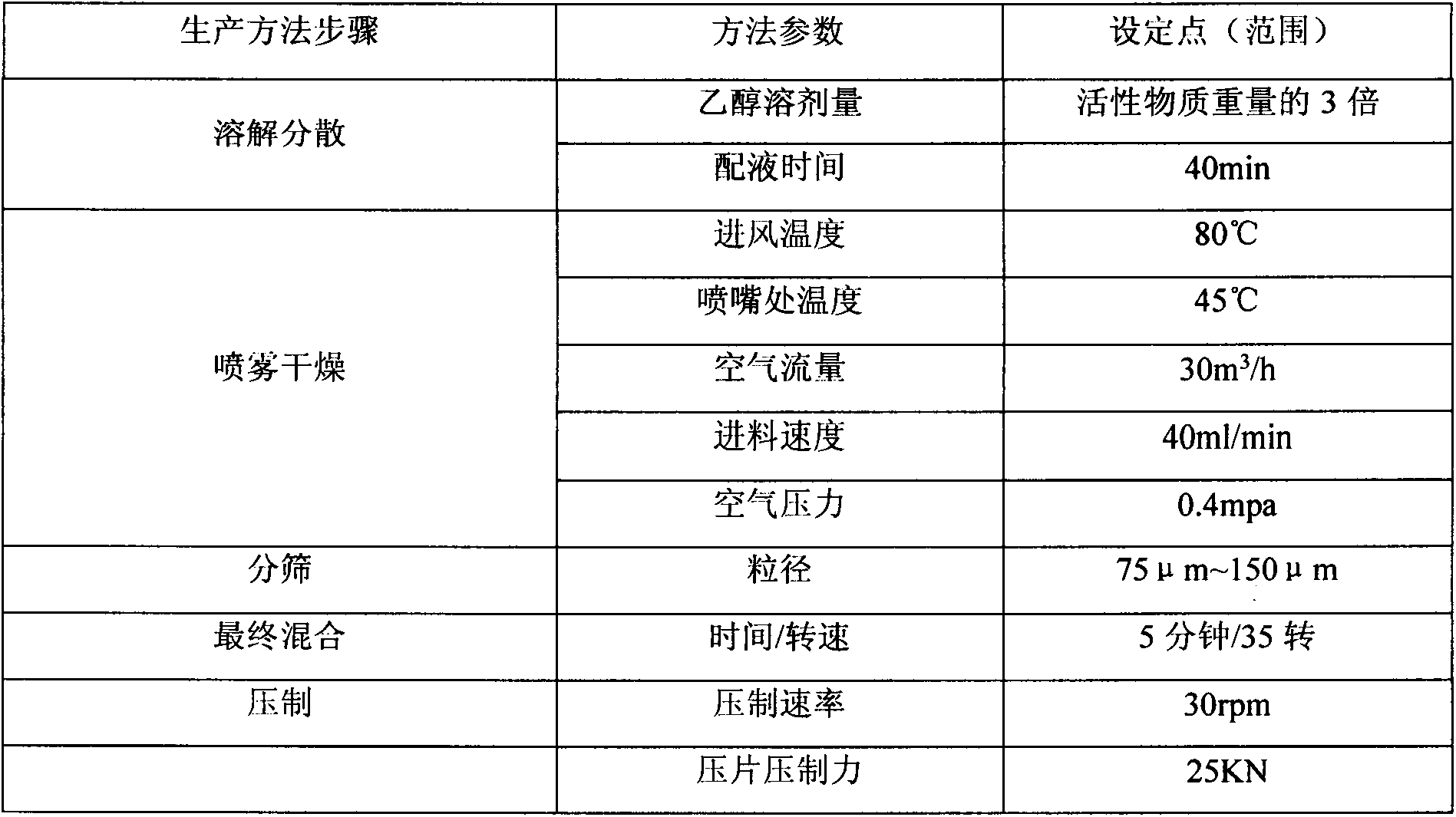
[0021] Add 50.0g of vildagliptin and 1.0kg of metformin hydrochloride to ethanol with 3 times the mass of the active substance and stir, then add 6g of magnesium aluminum silicate and 130g of hydroxypropyl cellulose to form a uniform dispersion. The prepared liquid is passed through a fluidized bed for spray drying, and the moisture content of the material is 1%. The dried material is passed through a vibrating sieve, sieved to select the powder containing the active substance with a particle size between 75 μm and 150 μm, and the powder containing the active substance is obtained by adding 1% magnesium stearate, and directly used for pressing into a tablet. The production process parameters are as follows:
...
Embodiment 2~6
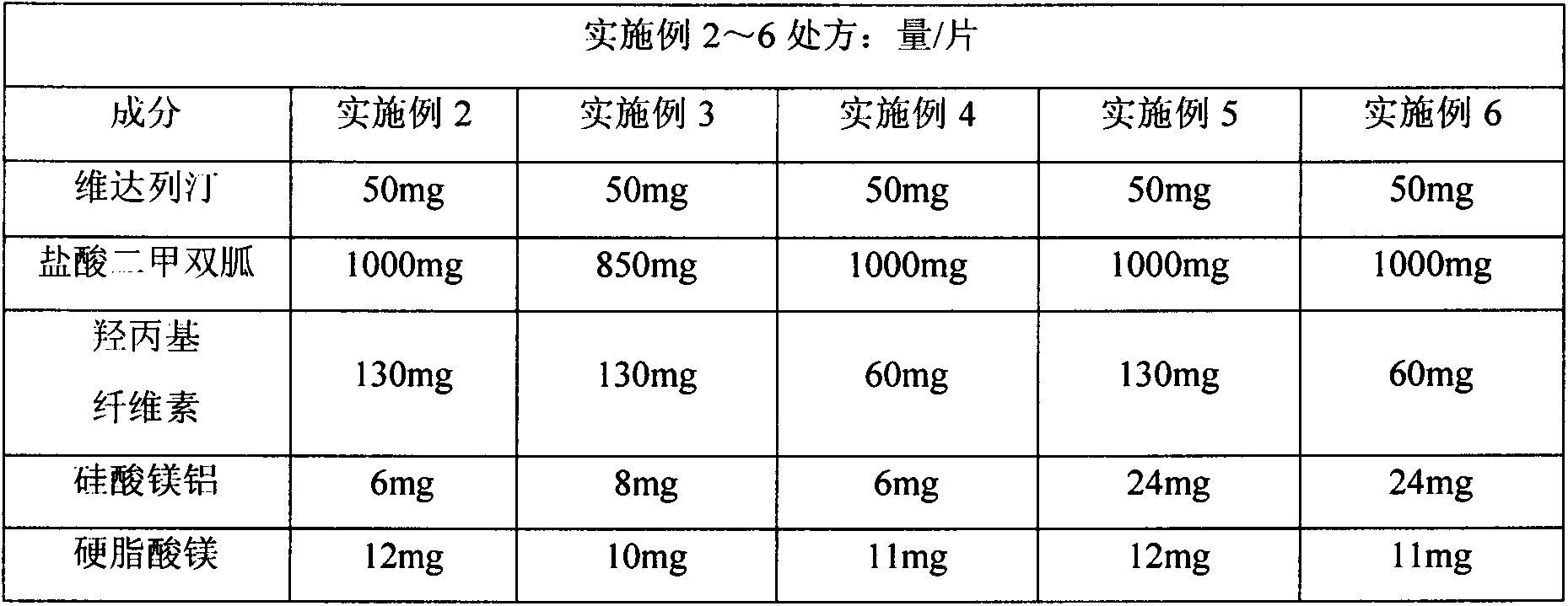
[0024]
[0025] production method:
[0026] Referring to the method of Example 1, vildagliptin and metformin hydrochloride were added to the solvent, and magnesium aluminum silicate and hydroxypropyl cellulose were added according to the prescription of Examples 2-6 to form a uniform dispersion. Referring to the process parameters of the following examples 2 to 6, spray drying is carried out through a fluidized bed, the moisture content of the material is 1%, and the powder containing the active substance is selected by sieving with a particle size between 75 μm and 150 μm. Magnesium steatate is then used directly for compression into tablets.
[0027]
Embodiment 11
[0032] Comparative example 1.1 production method:
[0033] Referring to the prescription of Comparative Example 1.1, vildagliptin, metformin hydrochloride and hydroxypropyl cellulose were added to ethanol to form a dispersion. Then, spray drying was carried out with reference to the process parameters in the preparation method of Example 1, and the moisture content of the material was 1%. Sieve to select the powder containing the active substance with a particle size between 75 μm and 150 μm obtained by the above method, add 1% magnesium stearate, and directly press it into a tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com