Human anti-CD26 antibody and applications thereof
A technology of CD26 and antibody, applied in the field of high-affinity fully human antibody preparation, to achieve the effect of inhibiting proliferation, invasion and metastasis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of CD26 extracellular region protein
[0062] The purpose of this study is to transfect eukaryotic cells with recombinant plasmids, G418 resistance screening to obtain stable transfected cell lines, secrete and express CD26 extracellular domain protein, and separate and purify CD26 extracellular domain protein by affinity chromatography.
[0063]The CD26 extracellular region gene is a synthetic gene selected from the "Extracellular" region of amino acid 29-766 in the Uniprot: P27487 sequence. The CD26 extracellular region gene is connected into the plasmid p3XFLAG-CMV9, and the restriction sites are Hind III, Xba I, Construction of recombinant plasmids (J. Sambrook. Molecular Cloning Experiment Guide. Third Edition. Science Press. 2002.P68).
[0064] Lipofectamine LTX Reagent (purchased from Invitrogen) was used to transfer the recombinant plasmid containing CD26 extracellular region gene into HLF cells according to the instructions. According to ...
Embodiment 2
[0066] Example 2 Isolation of fully human anti-CD26 single chain antibody
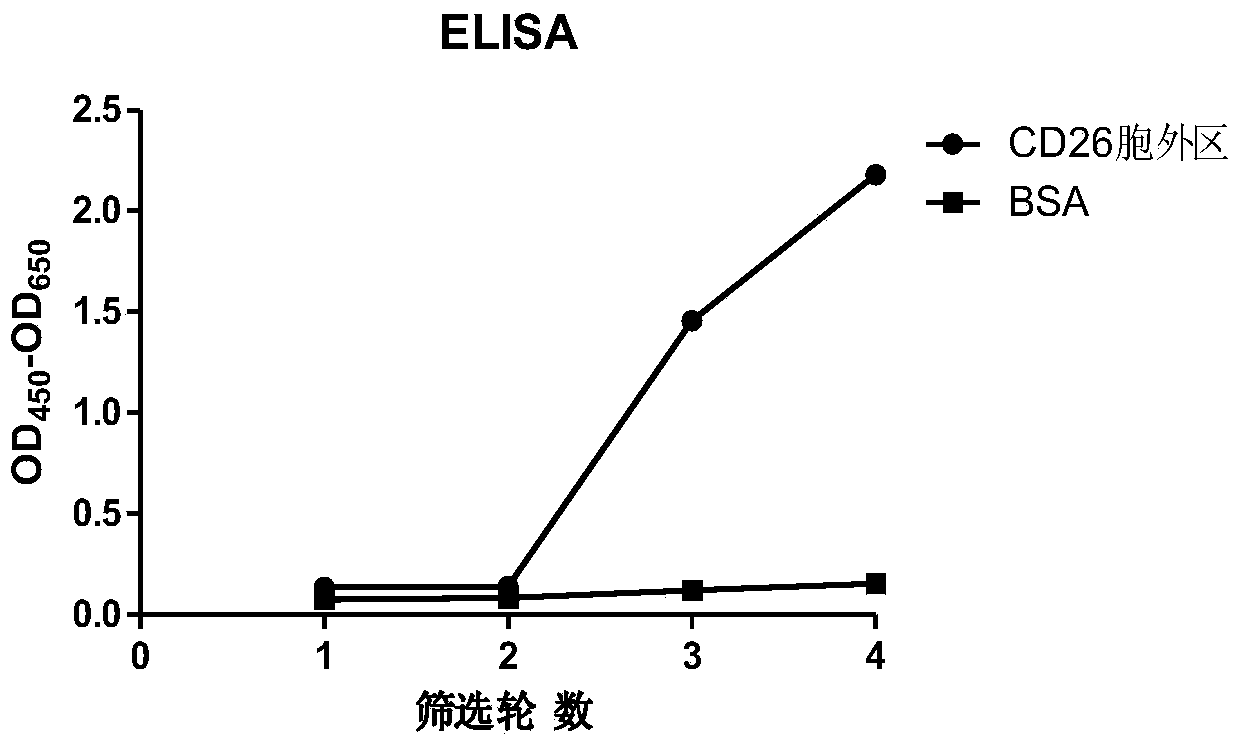
[0067] The single-chain antibody is obtained from a human single-chain antibody phage display library by solid-phase affinity screening of the CD26 extracellular region protein. The human single-chain antibody phage display library was constructed by Jiangsu Zhonghong Bioengineering Pharmaceutical Research Institute Co., Ltd. The phage display library containing the heavy chain and light chain variable regions of antibodies produced by human cells was constructed from human peripheral blood lymphocytes. The first round of amplification was carried out by using primers specific to the antibody variable region genes, and the second round of amplification was repeated. The variable regions of the light and chain chains were connected with a flexible linker peptide gene to form a single-chain antibody gene, and the single-chain antibody gene was cloned into the plasmid pHEN2 to transform Escherichia coli TG...
Embodiment 3
[0072] Example 3 Soluble expression and isolation and purification of anti-CD26 single chain antibody
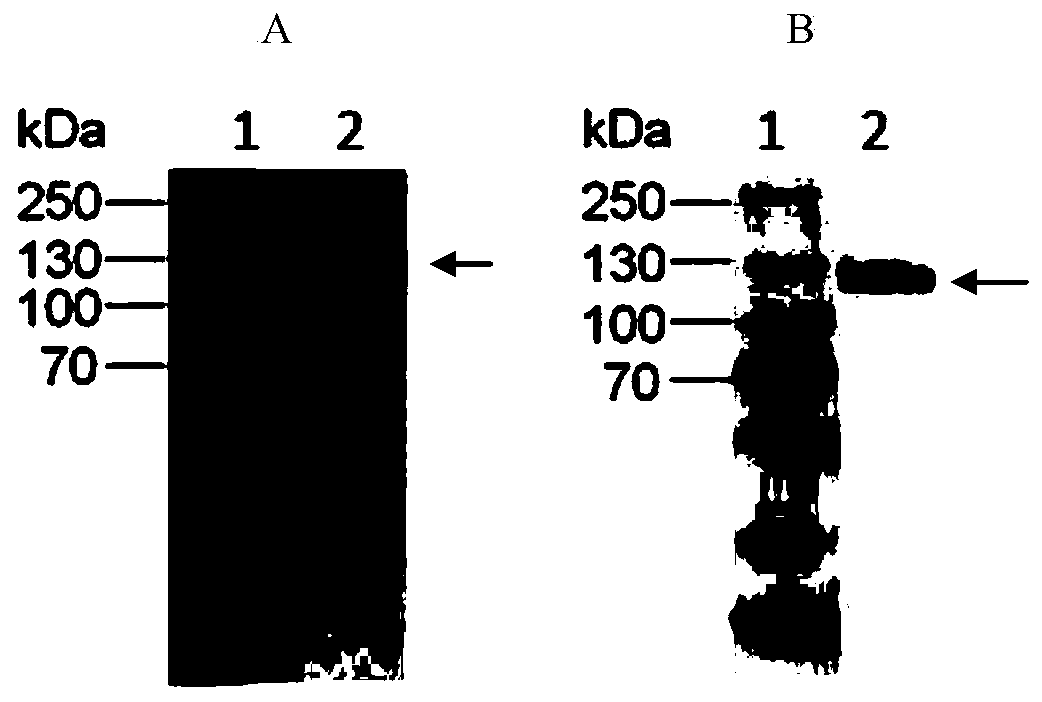
[0073] pHEN2 is a bifunctional phagemid vector with an amber stop codon (Amber) TAG between the expression detection tag (c-my tag) and the coat protein gene. If the phage is infected with an amber mutation (SupE) suppressor strain, such as TG1, the TAG codon is translated into glutamic acid, the sequence can be read through and translated, and the antibody fragment is fused to the coat protein p3 and expressed on the surface of the phage; when the phage is infected with a non-amber mutation suppressor For strains, such as HB2151, translation is terminated at TAG, and soluble antibody fragments can be obtained. The C-terminus of the antibody fragment has 6×His tag and c-myc tag, which is convenient for purification, detection and identification. ZHB-2cF9 single-chain antibody is soluble expressed in the periplasmic space of Escherichia coli, purified by high-osmotic method ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com