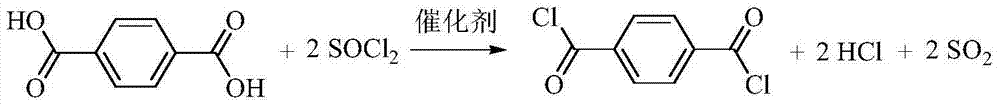
Method for preparing paraphthaloyl chloride
A technology of terephthaloyl chloride and terephthalic acid, which is applied in the field of chemical raw material preparation, can solve the problems of high cost and low catalyst activity, and achieve the effects of high product yield and purity, and simple and convenient reaction operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] 100 grams of terephthalic acid (PTA), 300 grams of thionyl chloride (SOCl 2 ) and 2 grams of 1-methylpyrrolidine were added to a 1-liter round-bottomed flask, stirred evenly, heated to 100°C for 10 hours, and the generated HCl and SO 2 The mixed gas is first absorbed with water, and then absorbed with 30% NaOH solution. After the reaction is completed, the catalyst and excess SOCl are recovered by distillation 2 156 grams, and 110 grams of product terephthaloyl chloride (TPC) were obtained by rectification under reduced pressure, and the separation yield was 90%. The product was detected by GC-MS and 1 H NMR, 13 C NMR was used for qualitative analysis, and gas chromatography detected that the purity was higher than 99%.
Embodiment 2~25
[0021] The specific method of Examples 2-25 is similar to that of Example 1, and the specific reaction conditions and results are listed in Table 1.
Embodiment 26
[0023] With the SOCl that embodiment 1 reclaims 2 and catalyst into a 1 L round bottom flask, followed by SOCl 2 145 grams, stirred evenly, raised the temperature to 100°C and reacted for 10 hours, the generated HCl and SO 2 The mixed gas is first absorbed with water, and then absorbed with 30% NaOH solution. After the reaction is completed, the catalyst and excess SOCl are recovered by distillation 2 156 grams, and obtain product TPC110 grams by rectification under reduced pressure, and separation yield is 90%. The product was detected by GC-MS and 1 H NMR, 13 C NMR was used for qualitative analysis, and gas chromatography detected that the purity was higher than 99%.
[0024] According to Example 1, it can be seen that 1-methylpyrrolidine can catalyze PTA and SOCl 2 The reaction prepares TPC, and the product yield can reach 90%. Examples 2-3 show that with 1-methylpiperidine and 1-ethylpiperidine as catalysts, the TPC yield can also reach 90%. Embodiment 4 does not us...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com