Injectable hollow hydroxyapatite microsphere/chitosan composite drug carrier material and preparation method thereof
A technology of hollow hydroxyapatite and empty hydroxyapatite, which is applied in the field of biomedical materials, can solve the problems of short release period and drug burst release, achieve good mechanical properties and improve the effect of sustained release performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
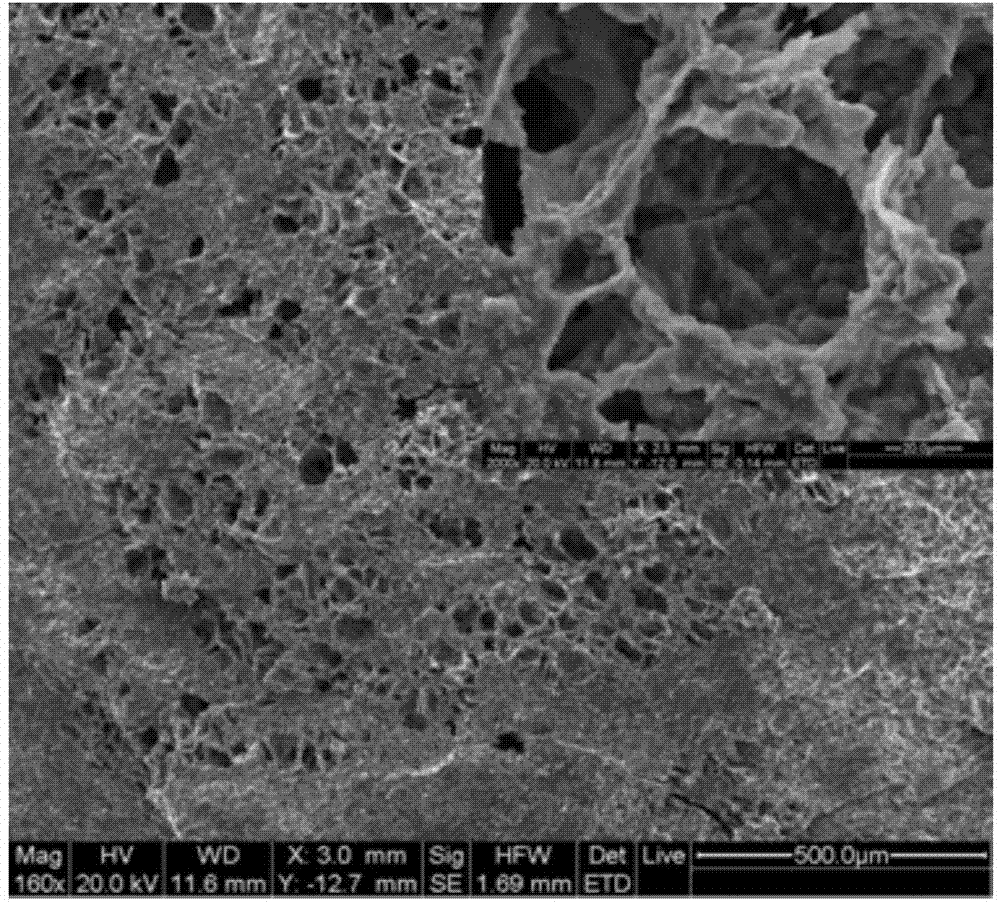
[0028] In a hollow hydroxyapatite microsphere / chitosan composite drug carrier material: the dispersed phase is the hollow hydroxyapatite microsphere, and the continuous phase is the hydrogel prepared by chitosan and beta-glycerophosphate sodium. The preparation process of the composite drug carrier is as follows:
[0029] (1) Preparation of hollow hydroxyapatite microspheres: weigh 7.4g Li 2 CO 3 , 10gCaCO 3 and 97.6 g H 3 BO 3 Powder, mix thoroughly in a mortar. The mixed powder was placed in a platinum crucible, and heated and melted for 30 minutes in a silicon carbide rod high-temperature furnace at 1100°C. Quenching and quenching the molten glass liquid to produce borate glass, in which Li 2 O, CaO, B 2 o 3 The molar ratios are 10%, 10% and 80%, respectively. After breaking the glass block, glass particles with an average particle size of 2-5 μm are obtained. The sieved glass particles were sprayed into a tube furnace at 700°C to make spherical borate glass micro...
Embodiment 2
[0035] The preparation process of the composite drug carrier carrying lysozyme is as follows:
[0036] (1) Preparation of hollow hydroxyapatite microspheres: weigh 7.4g Li 2 CO 3 , 10gCaCO 3 and 97.6 g H 3 BO 3 Powder, mix thoroughly in a mortar. The mixed powder was placed in a platinum crucible, and heated and melted for 30 minutes in a silicon carbide rod high-temperature furnace at 1100°C. Quenching and quenching the molten glass liquid to produce borate glass, in which Li 2 O, CaO, B 2 o 3 The molar ratios are 10%, 10% and 80%, respectively. After breaking the glass block, glass particles with an average particle size of 2-5 μm are obtained. The sieved glass particles were sprayed into a tube furnace at 700°C to make spherical borate glass microspheres. Weigh 1g of spheroidized glass microspheres and add to 100ml of K with a concentration of 0.25mol / L and a pH of 9.0 2 HPO 4 solution, put it in a constant temperature box at 25°C, and take it out after soaking ...
Embodiment 3
[0041] The preparation process of the composite drug carrier carrying vancomycin is as follows:
[0042] (1) Preparation of hollow hydroxyapatite microspheres: weigh 7.4g Li 2 CO 3 , 10gCaCO 3 and 97.6 g H 3 BO 3 Powder, mix thoroughly in a mortar. The mixed powder was placed in a platinum crucible, and heated and melted for 30 minutes in a silicon carbide rod high-temperature furnace at 1100°C. Quenching and quenching the molten glass liquid to produce borate glass, in which Li 2 O, CaO, B 2 o 3 The molar ratios are 10%, 10% and 80%, respectively. After breaking the glass block, glass particles with an average particle size of 2-5 μm are obtained. The sieved glass particles were sprayed into a tube furnace at 700°C to make spherical borate glass microspheres. Weigh 1g of spheroidized glass microspheres and add to 100ml of K with a concentration of 0.25mol / L and a pH of 9.0 2 HPO 4 solution, put it in a constant temperature box at 25°C, and take it out after soakin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com