Injection alprostadil freeze-dried emulsion
A technology of alprostadil and freeze-dried emulsion, which is applied in the field of alprostadil freeze-dried emulsion for injection, can solve the problems of toxic and side effects, carcinogenicity, etc., and achieve the effects of prolonging the validity period, improving the quality of the preparation, and improving the safety of injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
[0044]
[0045] Preparation Process
[0046] 1) Add 1g of egg yolk lecithin to 3g of soybean oil at 70°C under nitrogen protection, and stir to dissolve;
[0047] 2) After the dissolution is complete, add 0.0005g alprostadil to dissolve completely and form an oil phase;
[0048] 3) Dissolve 2.5g glycerin in the water phase, and adjust the pH to 5 with sodium citrate to form the water phase;
[0049] 4) Put the water phase under nitrogen protection conditions at 70°C, add the oil phase to the water phase under high-speed shearing conditions, and shear for 5 minutes to form colostrum;
[0050] 5) Cool the colostrum to room temperature, and dilute to 100ml with water for injection;
[0051] 6) Homogenize the colostrum under 800-900 bar pressure for 5 minutes, and cool to room temperature;
[0052] 7) Add 10g of lactose and 1g of arginine, stir to dissolve and mix evenly, perform sterile filtration with a 0.22um filter, remove water according to a certain freeze-dr...
Embodiment 2
[0054]
[0055] Preparation Process
[0056] 1) Add 2.5g of egg yolk lecithin to 20g of soybean oil at 70°C under nitrogen protection, and stir to dissolve;
[0057] 2) After the dissolution is complete, add 0.01g alprostadil to dissolve completely and form an oil phase;
[0058] 3) Dissolve 2.5g glycerin in the water phase, and adjust the pH to 6 with sodium citrate to form the water phase;
[0059] 4) Put the water phase under nitrogen protection conditions at 70°C, add the oil phase to the water phase under high-speed shearing conditions, and shear for 5 minutes to form colostrum;
[0060] 5) Cool the colostrum to room temperature, homogenize for 5 minutes under 800-900 bar pressure, and cool to room temperature;
[0061] 6) Dissolve 10g of lactose and 1g of histidine in a small amount of water for injection, add it to the emulsion, mix well, and then adjust the volume to 100ml, perform sterile filtration with a 0.22um filter, remove moisture according to a certain fre...
Embodiment 3
[0063]
[0064] The preparation process is the same as in Example 1.
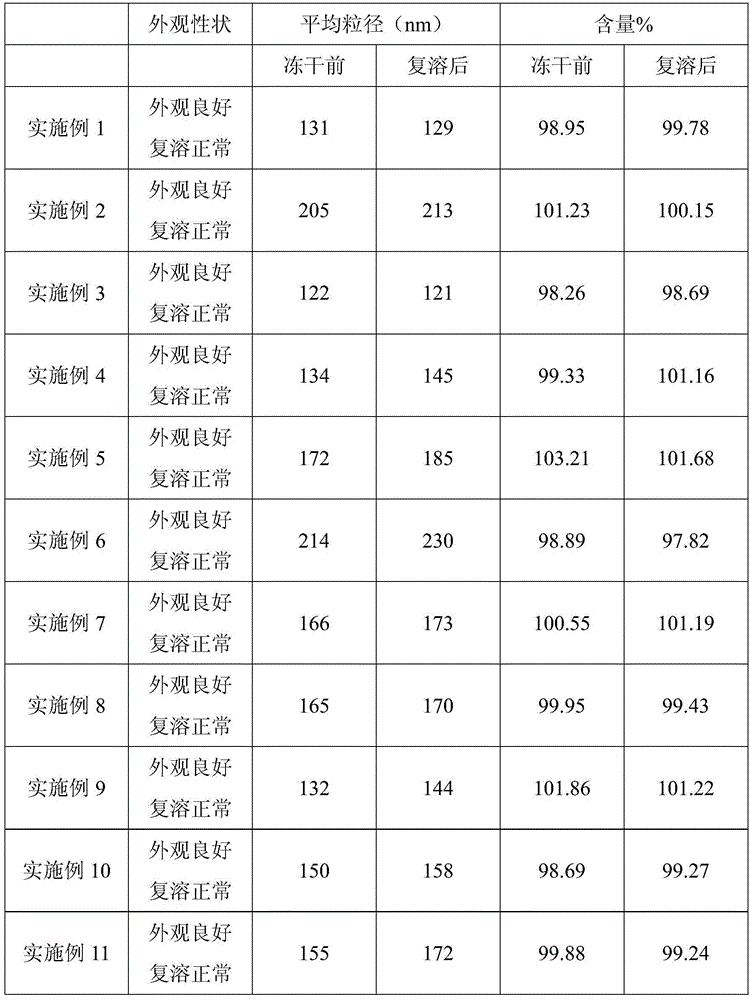
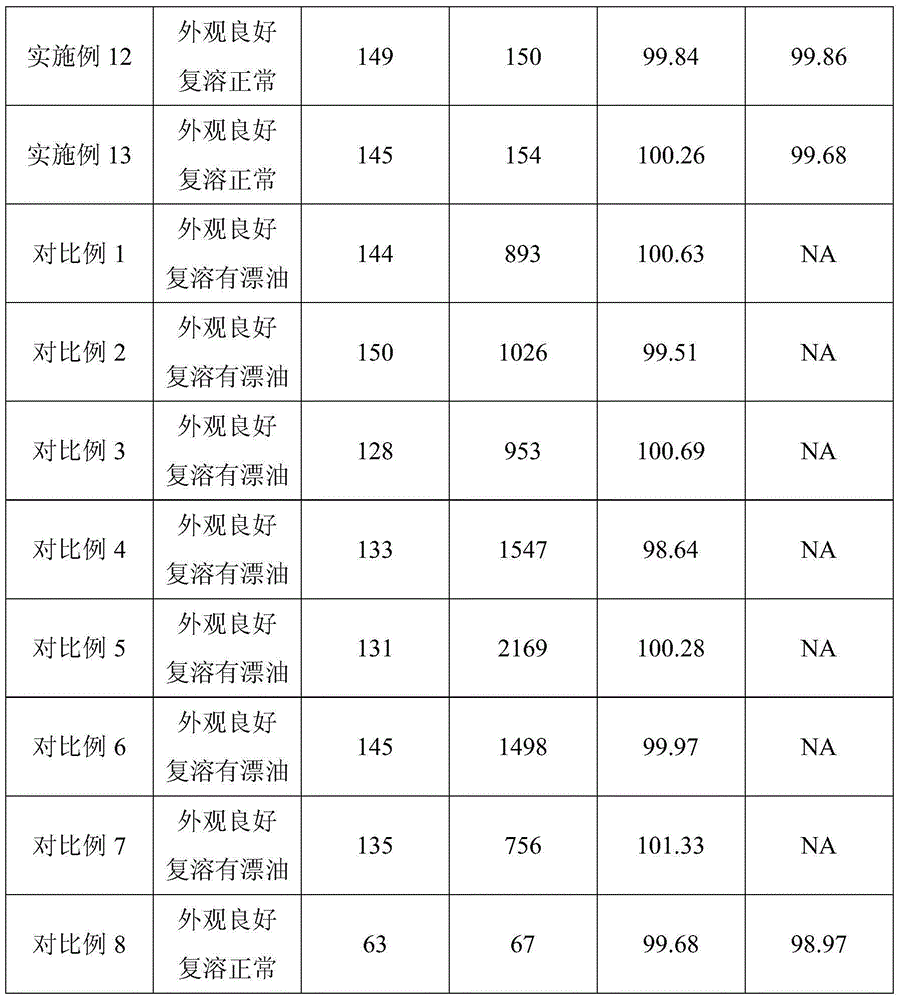
[0065] Alprostadil freeze-dried emulsion prepared with different oil types and contents for injection
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com