Hypoglycemic peptide and drug use thereof
A hypoglycemic and drug technology, applied in the field of medicine, can solve problems such as serious adverse reactions, inconvenient insulin administration, hypoglycemia, etc., and achieve the effect of avoiding gastrointestinal digestion and helping gastrointestinal absorption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Synthesis of embodiment 1 hypoglycemic peptide
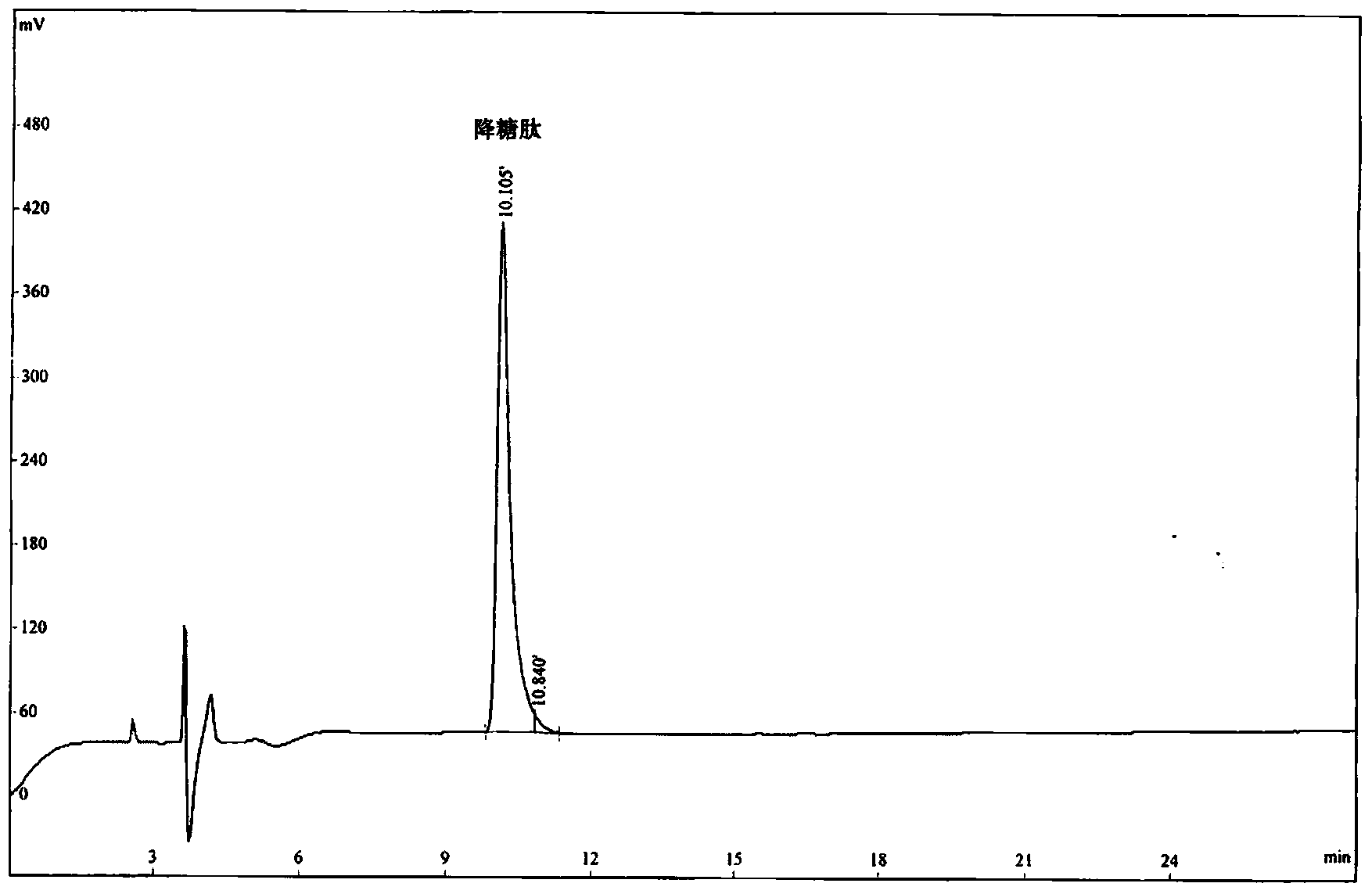
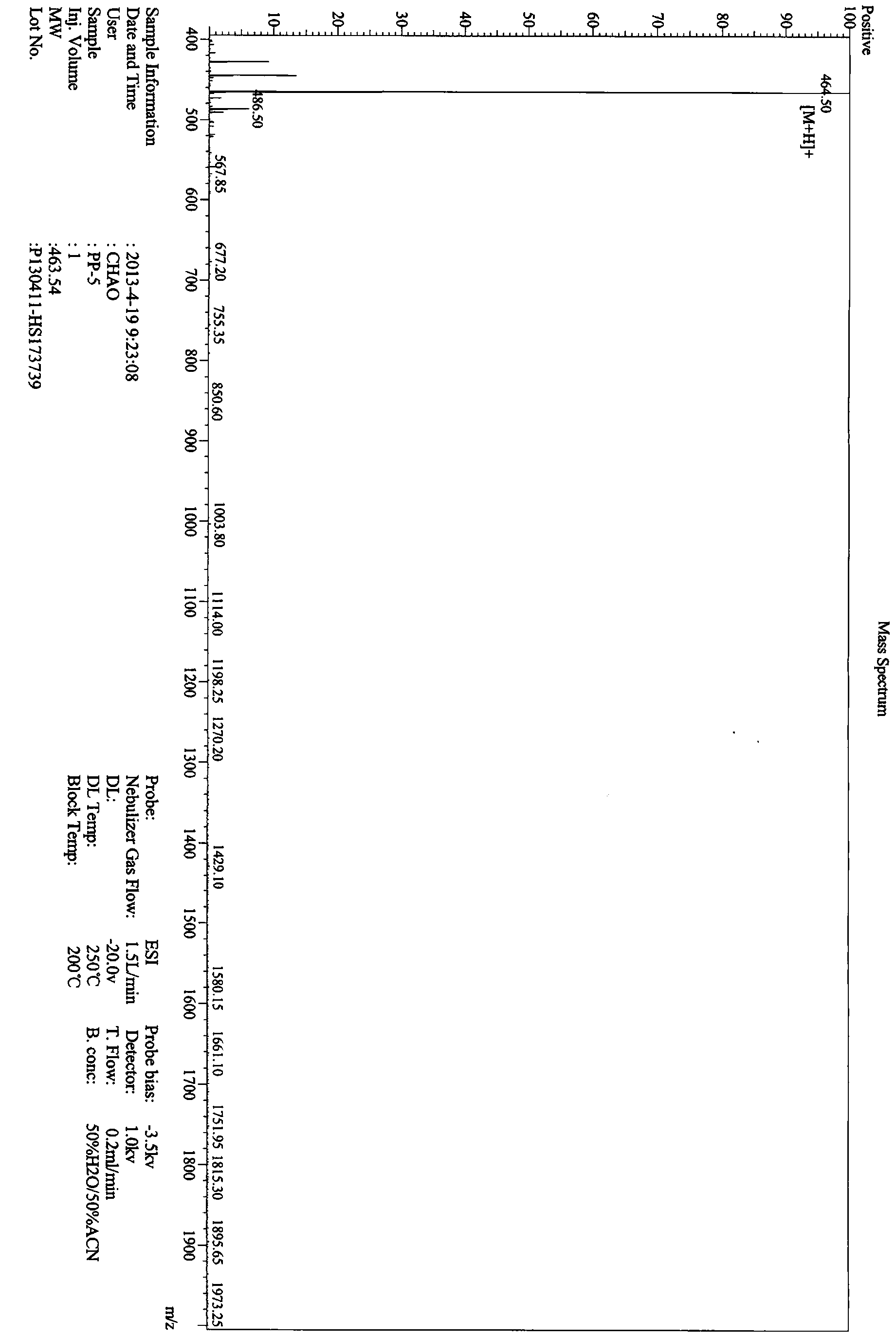
[0058] The hypoglycemic peptide of the present invention was synthesized by the polypeptide solid-phase synthesis method of Jill Biochemical (Shanghai) Co., Ltd. with a polypeptide synthesizer. The peptide synthesis starts from H-Pro-2-Chlorotrityl chloride Resin, using HOBt / DCC as the activator, followed by Fmoc-Gly-OH, Fmoc-Pro-OH, Fmoc-Pro-OH, Fmoc-Pro-OH. After each amino acid reacts, it is fully washed by DMF, piperidine is deprotected by Fmnoc, and the DMF is fully washed again before the next amino acid is connected. After all the synthesis is completed, use TFA:H 2 O:EDT=95:2.5:2.5 (v / v) Cut the hypoglycemic peptide from the resin, precipitate it with glacial ether, wash it, and use a C18 column for RP-HPLC purification. Chromatographic conditions: Xbrige BEH130C18 (4.6×250mm, 5μm), reagent A: 0.1% trifluoroacetic acid in acetonitrile; reagent B: 0.1% trifluoroacetic acid in water. The flow rate is 1.0ml / min, a...
Embodiment 2
[0061] Embodiment 2 animal experiment grouping and administration
[0062] Take 30 KK-Ay mice, half male and half female, the blood glucose of female mice is not lower than 7.8mmol / L, and the blood glucose of male mice is not lower than 11.1mmol / L, and they are randomly divided into 5 groups with SPSS11.0 software, each group has 6 Only, so that there is no significant difference in blood glucose in each group, they are: Group B (model control group), Group C (positive control group), Group D (low-dose hypoglycemic peptide group), group E (medium-dose hypoglycemic peptide group) , Group F (high-dose hypoglycemic peptide group). Another 6 normal ICR mice were selected as group A (normal reference group). Group C was given rosiglitazone 8 mg / kg, and groups D, E, and F were given hypoglycemic peptides 3 mg / kg, 10 mg / kg, and 30 mg / kg, respectively. Groups A and B were given an equal volume of normal saline. All groups were intragastrically administered for 2 weeks.
Embodiment 3
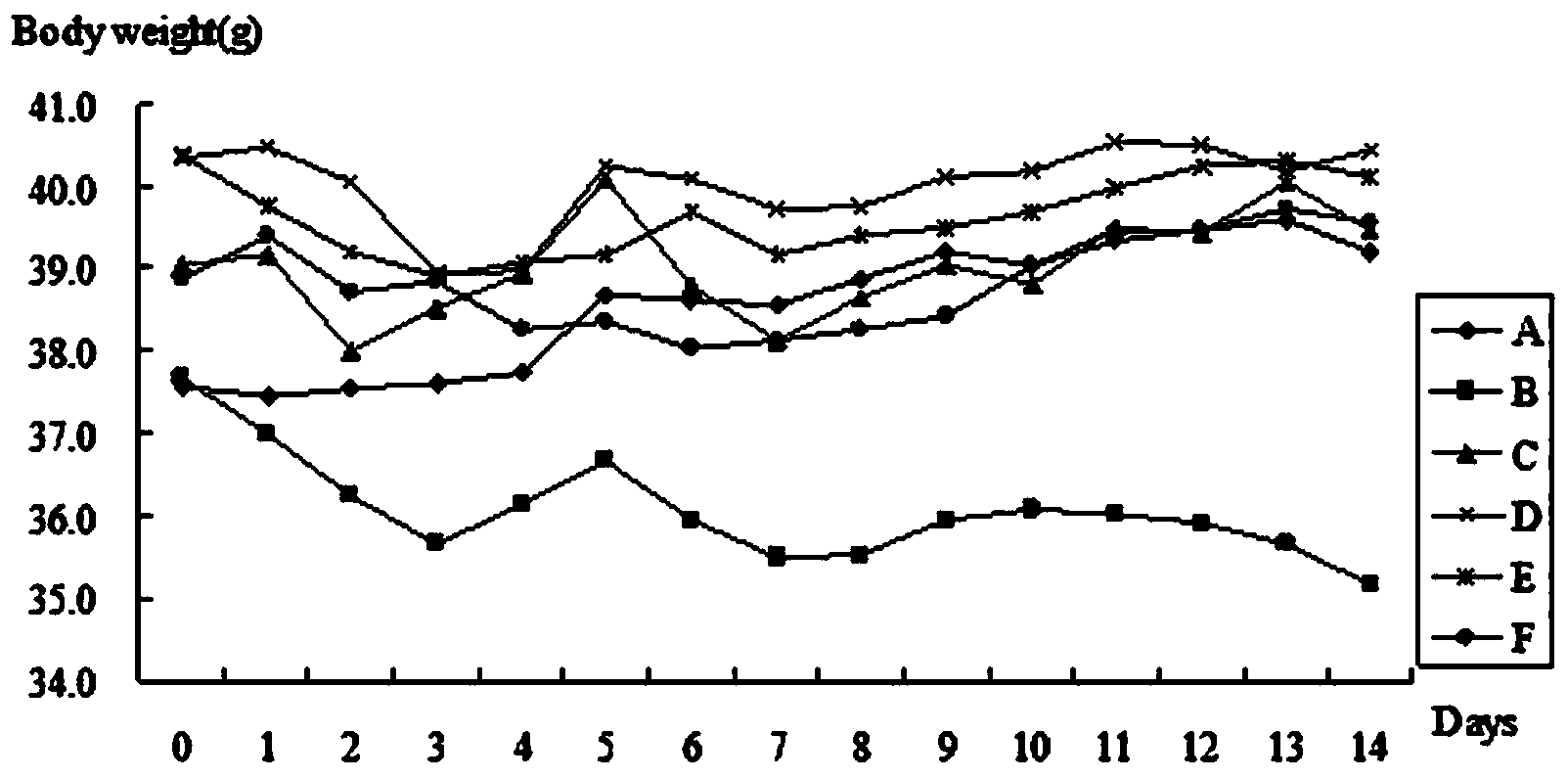
[0063] The effect of embodiment 3 hypoglycemic peptide on the body weight of KK-Ay mice
[0064] Each group of KK-Ay mice described in embodiment 2 weighed once every day, and counted once every 7 days, and each group's body weight was compared with before administration, A,
[0065] The body weights of groups C, D, and E were compared with those of groups B and C, and the trend of body weight changes was observed.
[0066] During the administration, the effect of hypoglycemic peptide on the body weight of KK-Ay mice is shown in Table 1 and image 3 shown.
[0067] Table 1 Effects of hypoglycemic peptides on the body weight of KK-Ay mice
[0068]
[0069] N=6, *P<0.05,**P<0.01. Group A,C,D,E are compared with Group B,respectively.
[0070] It can be seen from the tables and charts that the body weight of each administration group (C, D, E, F) is higher than that of B (model group), and the body weight of group D > group E > body weight of group F tends to appear in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com