A kind of molecular probe and its preparation method and application
A molecular probe and probe technology, used in chemical instruments and methods, pharmaceutical formulations, radioactive preparations in vivo, etc., can solve the problems of false positives and false negatives in disease diagnosis, and the inability of single-target molecular imaging to accurately reflect biological processes in vivo. , to achieve the effect of good stability and high imaging contrast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
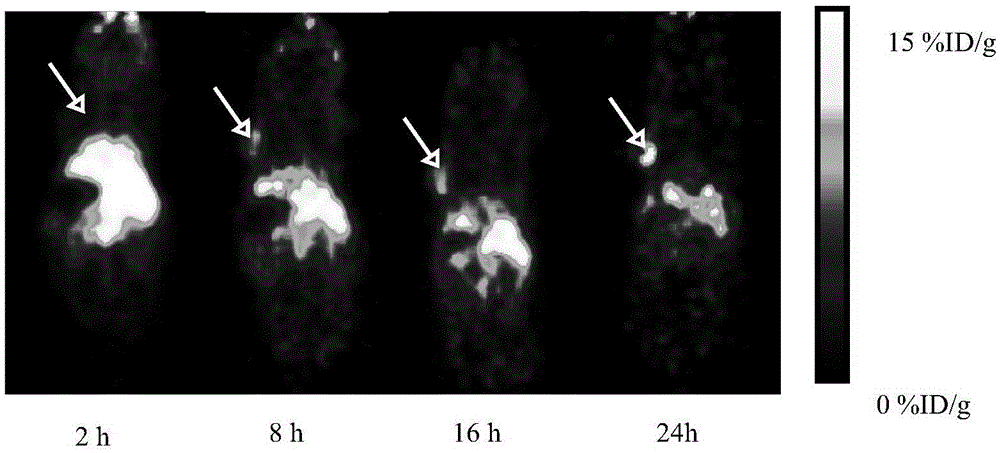
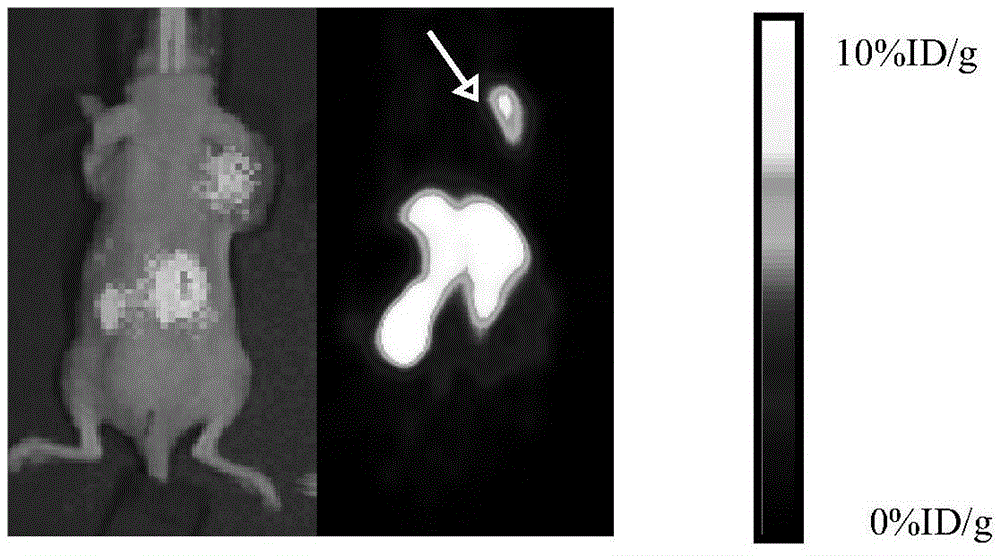
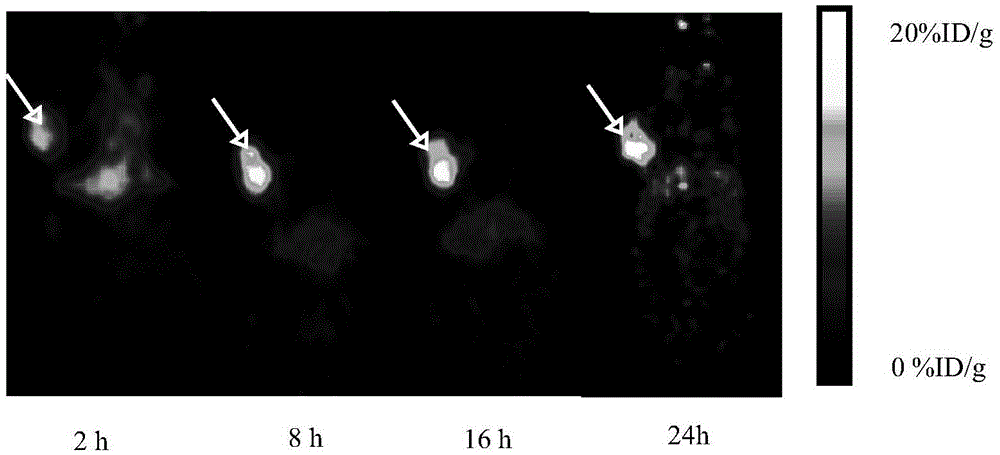
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of Molecular Probe D1
[0043] 1) Under nitrogen protection, the substance Diamsar (20 mg, 63.7 μmol) was dissolved in acetone-methanol mixed solvent (wherein by volume ratio, V 丙酮 :V 甲醇 =1:2), then added p-bromophenethylamine (60mg, 300μmol), reacted at 60-70℃ for 10h, cooled to room temperature, purified by HPLC preparative chromatography, and freeze-dried to obtain the product A1 (25.3mg, 72 %, the retention time is 8.7min). A1 electrospray mass spectrometer ESI-MS result: m / z=552.8 (A1 molecular formula is C 30 h 52 N 10 , the theoretical value is 552.8).
[0044]
[0045]2) Dissolve 4-(N-maleimidomethyl)cyclohexane-1-carboxylic acid sulfosuccinimidyl ester sodium salt (Sulfo-SMCC; 43.6 mg, 100 μmol) in EDC (28.8 mg , 150μmol) / NHS (16.2mg, 140μmol) mixed solution (EDC: 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride; NHS: N-hydroxysuccinimide ), using 0.1mol L -1 Dilute hydrochloric acid to adjust the pH of the solution t...
Embodiment 2
[0051] Example 2 Preparation of Molecular Probe E2
[0052] 1) Under the protection of nitrogen, dissolve the substance Diamsar (20 mg, 63.7 μmol) in a mixed solvent of tetrahydrofuran and methanol (wherein, V 四氢呋喃 :V 甲醇 =1:2), then added β-alanine (35.6mg, 400μmol), reacted at 55°C for 12-20h, cooled to room temperature, purified by HPLC preparative chromatography, and freeze-dried to obtain the product A2 (23.8mg , 82%, retention time is 7.5min). A2 electrospray mass spectrometer ESI-MS result: m / z=456.4 (A2 molecular formula is C 20 h 44 N 10 o 2 , theoretical m / z value is 456.4).
[0053]
[0054] 2) Dissolve the obtained product A2 (5.5mg, 10μmol) in 3mL boric acid buffer (pH 8.5); then add 4-(N-maleimidomethyl)cyclohexane-1-carboxylic acid Sodium salt of succinimide ester (Sulfo-SMCC; 34.88 mg, 80 μmol) was dissolved in EDC (20.16 mg, 90 μmol) / NHS (10.5 mg, 90 μmol) mixed solution (EDC: 1-(3-dimethylaminopropyl )-3-ethylcarbodiimide hydrochloride; NHS: N-hydro...
Embodiment 3
[0062] The preparation of embodiment 3 molecular probe G3, H3
[0063] 1) Under the protection of nitrogen, dissolve the compound of formula II (Diamsar: 20 mg, 63.7 μmol) in a mixed solvent of tetrahydrofuran and acetone (wherein by volume, V 四氢呋喃 :V 丙酮 =2:1), then Fmoc-protected 3,4,5-trihydroxy-2-carboxy-7-aminopyran (structural formula below) (85.75 mg, 200 μmol) was dissolved in EDC (13.44 mg, 70 μmol) with HOBT (70μmol) mixed solution, mix the two solutions, react at room temperature for 2-4 hours, add morpholine (100μmol), and then stir at 50-60℃ for 8-9 hours, and finally use 0.1mol L -1 Dilute hydrochloric acid solution to adjust the pH to 5-7, react at 40-50°C for 2-3 hours, cool to room temperature, prepare HPLC for separation and purification, and lyophilize to obtain product A3 (33.5mg, 76%, retention time: 6.9min) . A3 electrospray mass spectrometer ESI-MS result: m / z=692.8 (A3 molecular formula is C 28 h 56 N 10 o 10 , theoretical m / z value is 692.8).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com