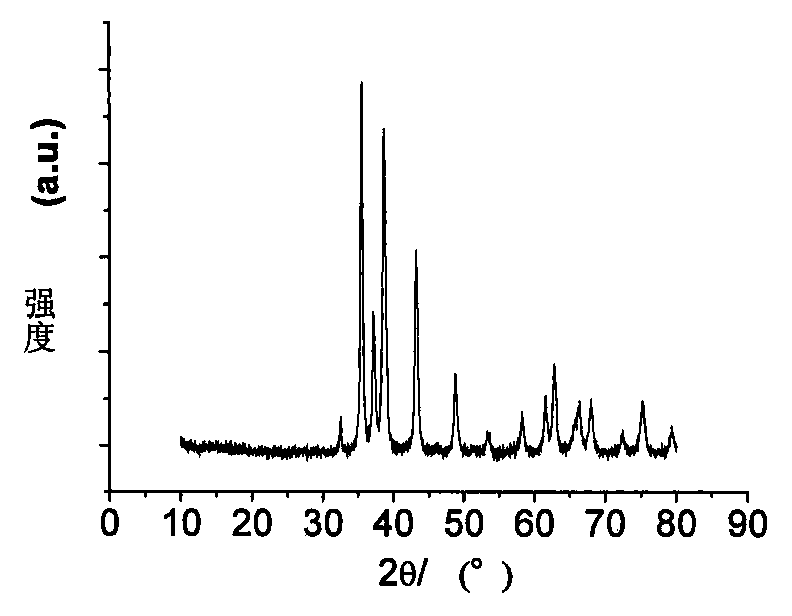
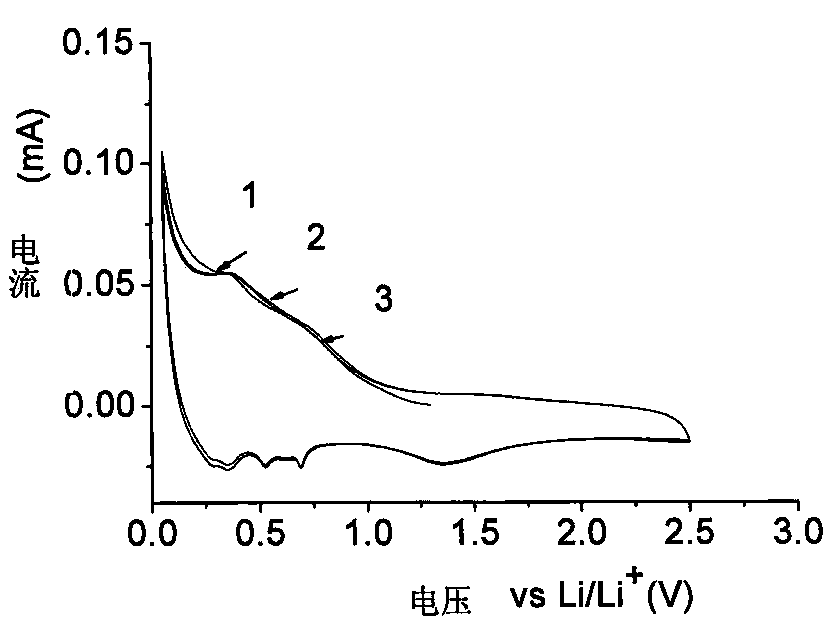
Preparation method of nano cobalt-zinc composite oxide
A composite oxide, nano-cobalt technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problem of high cost, affecting the practical application of metal oxide anode materials, poor cycle performance, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] a. Dissolve 1 g of tetrabutylammonium bromide in a mixture of 1.5 ml of n-amyl alcohol and 30 ml of cyclohexane, stir for 30 minutes, and then add a mixture of zinc acetate and cobalt acetate in a volume ratio of 1:1 after stirring and mixing solution, wherein the concentration of zinc acetate is 0.3mol / L, and the concentration of cobalt acetate is 0.1mol / L;
[0018] b. Add dropwise a concentration of 3 mol / LKOH solution to the mixed solution obtained in step a, adjust pH 7.5-8.5 until the solution is slightly alkaline, and precipitation occurs, then transfer the solution containing the precipitation into an autoclave, control The reaction temperature was 130°C, and the heating time was 6h;
[0019] c. Then at a temperature of 170°C, heat for 24h, filter to obtain a precipitate, wash the precipitate to neutrality, and then transfer it to a muffle furnace, control the temperature at 500°C, and heat for 6h to obtain black nano-cobalt zinc. complex oxide.
Embodiment 2
[0021] a. Dissolve 1 g of tetrabutylammonium bromide in a mixture of 1.5 ml of n-amyl alcohol and 30 ml of cyclohexane, stir for 30 minutes, and then add a mixture of zinc acetate and cobalt acetate in a volume ratio of 1:1 after stirring and mixing solution, wherein the concentration of zinc acetate is 0.3mol / L, and the concentration of cobalt acetate is 0.1mol / L;
[0022] b. Add dropwise a concentration of 3 mol / LKOH solution to the mixed solution obtained in step a, adjust pH 7.5-8.5 until the solution is slightly alkaline, and precipitation occurs, then transfer the solution containing the precipitation into an autoclave, control The reaction temperature was 140°C, and the heating time was 8h;
[0023] c. Then heat for 24h at a temperature of 170°C, filter to obtain a precipitate, wash the precipitate to neutrality, and then transfer it to a muffle furnace, control the temperature at 600°C, and heat for 10h to obtain black nano-cobalt zinc. complex oxide.
Embodiment 3
[0025] a. Dissolve 1 g of tetrabutylammonium bromide in a mixture of 1.5 ml of n-amyl alcohol and 30 ml of cyclohexane, stir for 30 minutes, and then add a mixture of zinc acetate and cobalt acetate in a volume ratio of 1:1 after stirring and mixing solution, wherein the concentration of zinc acetate is 0.3mol / L, and the concentration of cobalt acetate is 0.1mol / L;
[0026] b. Add dropwise a concentration of 3 mol / LKOH solution to the mixed solution obtained in step a, adjust pH 7.5-8.5 until the solution is slightly alkaline, and precipitation occurs, then transfer the solution containing the precipitation into an autoclave, control The reaction temperature was 150°C, and the heating time was 10h;
[0027] c. Then at a temperature of 170°C, heat for 24h, filter to obtain a precipitate, wash the precipitate to neutrality, and then transfer it to a muffle furnace, control the temperature at 700°C, and heat for 12h to obtain black nano-cobalt zinc. complex oxide.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com