Novel synthesis method of Ulipristal acetate
A technology of Eulyst acetate and a synthesis method, applied in the directions of steroids, organic chemistry, etc., can solve the problems of increased α addition ratio, increased steric hindrance, long steps, etc., and achieves simplified operation and low cost. , cleverly conceived effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
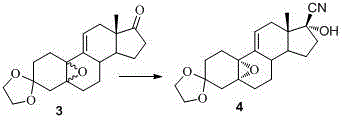
Embodiment 1
[0060] Example 1 compound 3 Synthesis
[0061]
[0062] Add 11.5g of disodium hydrogen phosphate dodecahydrate into dichloromethane, add 8.8mL of hexafluoroacetone and 15.8mL of 30% hydrogen peroxide in turn, stir at 0°C for 1 hour, add 10g of the compound 2 , the solution was orange-yellow, and continued to stir at 0°C for 18 hours. TLC monitored that the reaction was complete. Add 50 mL of 10% sodium sulfite to the reaction solution, stir for 15 minutes, separate the dichloromethane layer, and then wash the water layer with 2*30 mL of dichloromethane Extract, combine the dichloromethane layers, wash with 10% sodium sulfite (2*50mL), wash with water (2*50mL), dry with 20g of anhydrous sodium sulfate, and concentrate to obtain 10.6g of pale yellow foamy solid. (Theory: 10.5g yield is 100%) HPLC measured 5α, 10α / 5β, 10β as 81:19. 1 HNMR {400 MHz, CDCl 3 (TMS), δ (ppm)}: 0.874 (s, 3H, CH 3 ), 1.214-2.509 (m, 18H), 3.876-3.966 (m, 4H, OCH 2 CH 2 O), 5β,10β: 5.860 (d,...
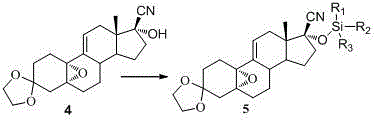
Embodiment 2
[0063] Example 2 compound 4 Synthesis
[0064]
[0065] 10g compound 3 After heating and dissolving with 20ml of acetone cyanohydrin, add 0.1ml of 1,8-diazabicyclo-bicyclo(5,4,0)-7-undecene (DBU) and stir at room temperature for 24h, a large amount of white solids precipitated , and then add 100ml of isopropyl ether and stir for 30min, then filter, wash the filter cake with isopropyl ether (2×20ml), collect the filter cake, and dry under reduced pressure at 50°C to obtain 5.95g (55%) of white solid. 1 HNMR {400 MHz, CDCl 3 (TMS), δ (ppm)}:0.935 (s, 3H, CH 3 ), 1.177~2.520 (m, 18H), 2.609 (s, 1H, OH), 3.882~3.969 (m, 4H, OCH 2 CH 2 O), 6.081 (t, 1H, J =2.8, =CH).
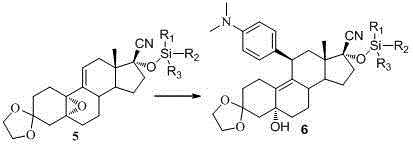
Embodiment 3
[0066] Example 3 Compound 5 Synthesis of (R 1 , R 2 , R 3 for methyl)
[0067]
[0068] 3.9g imidazole and 5g compound 4 After dissolving with 50ml of dry tetrahydrofuran, slowly add trimethylchlorosilane (5.5ml) dropwise at 0°C. After the addition is complete, continue to stir at this temperature for 3h. After the reaction is complete, a white needle-like solid precipitates out. Add 100ml of water , the solid was dissolved, extracted with ethyl acetate (3×40 ml), the combined ethyl acetate layers were washed with water (3×30 ml), dried over anhydrous sodium sulfate, and concentrated to give a white solid 6.1g (100%) mp: 160- 162°C. 1 HNMR {400 MHz, CDCl 3 (TMS), δ (ppm)}:0.219 (s, 9H, Si-CH 3 ), 0.879 (s, 3H, CH 3 ), 1.182~2.555 (m, 18H), 3.879~3.930 (m, 4H, OCH 2 CH 2 O), 6.082 (t, 1H, J =2.8, =CH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com